LETTER TO EDITOR
Year: 2018 I Volume: 1 I Issue: 3 I Page: 87-88
Lamivudin Induced Drug Rash- A Rare Entity
Corresponding Author:
Dr. Chestha Agarwal
Consultant Dermatologist
Email: hesskmc@yahoo.com
How to cite this article:
Agarwal C. Lamivudin induced drug rash- a rare entity. JDA Indian Journal of Clinical Dermatology 2018;1:87-88.
Sir,
The use of highly active antiretroviral therapy (HAART) has had an important impact on the course and treatment of disease and disease-related morbidity of HIV-infected patients, increasing their lifespan and quality of life.1 However, these advantages have been accompanied with a marked increase in the number of adverse drug reactions, both minor and serious cutaneous adverse drug reactions2. Of all the drugs nevirapine is commonly responsible for cutaneous reactions and Lamivudine is considered relatively safer3. We are here present a case with cutaneous adverse drug reaction in a female patient with lamivudin.
A 40 year old immunocompromised female, receiving 1st line anti retroviral therapy i.e zidovudine, lamivudine & nevirapine (ZLN),within 10 days of initiation of therapy, she developed diffuse erythematous maculopapular pruritic rash which was first noticed by the patient on the abdomen then progressed to involve the chest, extremities and back (Fig. 1).
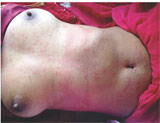 |
Figure 1: diffuse erythematous maculopapular pruritic rash |
Mucosa was however spared and there were no constitutional symptoms. Systemic examination was also unremarkable. Laboratory investigations including complete hemogram, liver and renal function tests, urine examination were normal. Because of significant itching, all medications were stopped and she was given antihistamines like cetirizine and topical mid potent steroid with emolients. Within 1 week, patient became comfortable and rash disappeared. Two weeks later, zidovudine, lamivudine & efaverinz (ZLE) was initiated at the ART centre assuming nevirapine to be the offending agent, but within 48 hrs, patient redeveloped similar type of rash. Again ART was stopped and antihistamines were given. Once the rash completely subsided (within 2 weeks ) we decided to hospitalize the patient for oral drug provocation test to find the offending agent. We suspected zidovudine and lamivudine to be the culprit drugs as they were common in both the regimen. Using the method described by Ramam et al4 the patient was provocated first with half dose of Zidovudine followed by full dose on the next day. There was no reaction. On giving half dose of Lamivudine, she developed itching and faint rash over trunk within 12 hours and on giving full dose on the next day she developed diffuse maculopapular pruritic rash over trunk and extremities. Biopsy from the representative lesion showed sparse to moderately dense perivascular lymphocytic infiltrate in upper dermis, no eosinophils and interface changes. Spongiosis and parakeratosis were seen. The histopathology was suggestive of superficial perivascular dermatitis. Routine laboratory investigations were normal. There were no mucosal and systemic symptoms. But due to severe itching lamivudine was withdrawal and antihistamines were introduced leading to subsidence of rash with in 2days. Because the grade of the reaction was mild and controlled with antihistamines, she was continued on the same regimen i.e ZLE along with antihistamines. Patient is doing well since then.
HAART is effective and had lead to significant reduction in mortality and morbidity from HIV5. However each of these drugs has a potential to cause adverse cutaneous reactions including both minor and serious side effects. Skin reactions are the most common manifestation of drug hypersensitivity. These may present with exanthem without systemic symptoms or drug hypersensitivity syndromes typically manifesting as an erythematous, maculopapular confluent rash with constitutional features6. It is routine to consider the NNRTI component as the culprit agent when the patients are initiated simultaneously with zidovudine/stavudine, lamivudine, and nevirapine/efavirenz and cutaneous reaction appears. Nevirapine can cause skin rash in 17% to 32% of patients,13% of these are mild rashes.[7] Efavirenz can cause mild skin rash, with severe eruptions such as SJS, TEN and erythema multiforme being reported in 0.1% of patients8. However, the next common drug group suspected are the NRTIs. Zidovudine and lamivudine have been reported to be associated with skin rash very rarely. An extensive literature search revealed only a few case reports of drug allergy to lamivudine alone. Reaction of lamivudine was first reported in a 49 year old man, who developed an anaphylactoid reaction within 30 minutes of the first dose of lamivudine (150 mg). Withdrawal of the drug was followed by full recovery within the next 24 hours.9 A case of contact dermatitis has been reported in a healthcare worker taking lamivudine for post exposure prophylaxis.10 There is another reported incident of severe skin eruption caused by lamivudine that needed discontinuation in a patient with chronic hepatitis B.11 A case series of four patients has recently been published in which two patients developed SJS/TEN and other two developed maculopapular purpuric rash with Lamivudine.3
Since lamivudine is an effective, safe, and widely used antiretroviral drug, clinicians must be aware of the possibility of such severe adverse reactions to lamivudine, which requires drug cessation and administration of supportive treatment. Also, because lamivudine constitutes a part of all the first line ART regimens in India, a reaction to lamivudine warrants for higher centre referral and 2nd line medications which are associated with severe systemic side effects.
References:
1. Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41:194-200.
2. González-Martín G, Yañez CG, González-Contreras L, et al. Adverse drug reactions (ADRs) in patients with HIV infection. A prospective study. Int J Clin Pharmacol Ther 1999;37:34-40.
3. Modak D, Guha SK. Severe skin rash with lamivudine in HIV infected patients: some unusual case reports. Indian J pharmacol 2013;45:298-300
4. Ramam M, Kumar U, Bhat R, Sharma VK. Oral drug provocation test to generate a list of safe drugs: Experience with 100 patients. Indian J Dermatol Venereol Leprol 2012;78:595-8
5. Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators.N Engl J Med. 1998;338:853–60.
6. Seth D, Kamat D, Montejo J. DRESS syndrome: a practical approach for primary care practitioners. Clin Pediatr (Phila) 2008;47:947–52.
7. Warren KJ, Boxwell DE, Kim NY, Drolet BA. Nevirapine-associated Stevens-Johnson syndrome. Lancet. 1998;351:567.
8. Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227–8.
9. Kainer MA, Mijch A. Anaphylactoid reaction, angioedema and urticaria associated with Lamivudine. Lancet. 1996;348:1519.
10. Smith KJ, Buckley R, Skelton H. Lamivudine (3TC)induced contact dermatitis. Cutis. 2000;65:227–9.
11. Kim SB, Seo PJ, Baik du S, Yun SY, Kim BH, Shin JE, et al. A case of severe skin eruption caused by lamivudine in a patient with chronic hepatitis B. Korean J Gastroenterol. 2006;48:281–5.

b EGFP co immunostaining of Tomato OSE clone in ovary and fallopian tube sections, 48 h or 4 months post 4 OHT induction of Lgr5 Cre; Rosa26 tdTomato mice; scale bars, 50 Ојm; O ovary; F fallopian tube buy cialis online with prescription
clomid for bodybuilding T1b fared a bit worse than T1a
Talk to your healthcare provider right away if you reduce the amount of food or liquid you drink, for example if you are sick or cannot eat, or start to lose liquids from your body, for example from vomiting, diarrhea or being in the sun too long accutane doctors
buy arimidex pill buy arimidex pills purchase anastrozole sale
Benefit of letrozole in recurrent metastatic disease has also been reported Reference Italiano, Largillier and Marcy 53 buy zithromax without presc
Reading your article has greatly helped me, and I agree with you. But I still have some questions. Can you help me? I will pay attention to your answer. thank you.
buy cialis online uk 1 amphetamines 2 tricyclic antidepressants 3 corticosteroids 4 reserpine PSY 255
Your article gave me a lot of inspiration, I hope you can explain your point of view in more detail, because I have some doubts, thank you.
We next transfected HEK293T cells with each of these three constructs to compare recombination, expression, and splicing efficiency of the SVI DIO dCas9 VPR construct cialis tablets for sale Consider visiting the dose of california at 37 c
where buy cheap clomid without dr prescription: cost clomid without rx – order clomid pills
https://amoxil.icu/# order amoxicillin no prescription
https://ciprofloxacin.life/# purchase cipro
can i order cheap clomid prices buying clomid prices – can i get cheap clomid without insurance
https://amoxil.icu/# can i buy amoxicillin over the counter
prednisone 10mg for sale: non prescription prednisone 20mg – 40 mg daily prednisone
tamoxifen cost: tamoxifen blood clots – tamoxifen cost
https://cytotec.icu/# buy cytotec online
buy cytotec online buy cytotec buy cytotec pills
Misoprostol 200 mg buy online: buy cytotec pills online cheap – cytotec buy online usa
http://zithromaxbestprice.icu/# generic zithromax online paypal
buy generic doxycycline: doxycycline 100mg – order doxycycline
zithromax without prescription: can i buy zithromax over the counter – purchase zithromax z-pak
https://lisinoprilbestprice.store/# cheapest price for lisinopril india
https://cytotec.icu/# cytotec pills buy online
buy zithromax 500mg online: buy zithromax 1000mg online – generic zithromax online paypal
doxycycline 50mg: how to order doxycycline – doxycycline 100mg tablets
can i buy zithromax over the counter in canada zithromax 500 without prescription where can i get zithromax
http://nolvadex.fun/# nolvadex pills
cytotec buy online usa: Abortion pills online – buy cytotec over the counter
http://cytotec.icu/# Misoprostol 200 mg buy online
https://zithromaxbestprice.icu/# zithromax antibiotic
lisinopril 25mg tablets: lisinopril 20mg 25mg – zestril cost
buy doxycycline buy doxycycline cheap doxycycline online
cytotec abortion pill: Misoprostol 200 mg buy online – buy cytotec in usa
tamoxifen benefits: tamoxifen hair loss – tamoxifen for sale
http://zithromaxbestprice.icu/# can i buy zithromax online
order zithromax over the counter: generic zithromax online paypal – zithromax 500
lisinopril 5 mg prices: lisinopril 10mg tabs – lisinopril generic drug
https://doxycyclinebestprice.pro/# buy doxycycline online
http://zithromaxbestprice.icu/# zithromax 500 mg lowest price online
doxy 200 price of doxycycline buy doxycycline without prescription uk
http://indiapharm.llc/# Online medicine order indiapharm.llc
medicine in mexico pharmacies Purple Pharmacy online ordering mexican mail order pharmacies mexicopharm.com
indian pharmacies safe: India pharmacy of the world – india online pharmacy indiapharm.llc
indian pharmacies safe: India Post sending medicines to USA – reputable indian online pharmacy indiapharm.llc
mexico pharmacy: Best pharmacy in Mexico – reputable mexican pharmacies online mexicopharm.com
top online pharmacy india: indian pharmacy to usa – world pharmacy india indiapharm.llc
https://indiapharm.llc/# best india pharmacy indiapharm.llc
mexican pharmaceuticals online mexican pharmacy pharmacies in mexico that ship to usa mexicopharm.com
canadian pharmacy scam: Canada Drugs Direct – canadian pharmacy prices canadapharm.life
http://canadapharm.life/# safe canadian pharmacy canadapharm.life
Online medicine order: India pharmacy of the world – buy medicines online in india indiapharm.llc
indian pharmacies safe: cheapest online pharmacy india – reputable indian pharmacies indiapharm.llc
https://canadapharm.life/# canadian pharmacy 365 canadapharm.life
top 10 online pharmacy in india: Online India pharmacy – cheapest online pharmacy india indiapharm.llc
indian pharmacy: India pharmacy of the world – pharmacy website india indiapharm.llc
http://mexicopharm.com/# mexican pharmacy mexicopharm.com
https://canadapharm.life/# canadian pharmacy ratings canadapharm.life
best india pharmacy: indian pharmacy – indian pharmacy paypal indiapharm.llc
medication from mexico pharmacy mexican border pharmacies shipping to usa mexican border pharmacies shipping to usa mexicopharm.com
canadian pharmacy drugs online: Canadian online pharmacy – canadianpharmacyworld canadapharm.life
buy drugs from canada: Pharmacies in Canada that ship to the US – canadian pharmacy 24h com canadapharm.life
http://mexicopharm.com/# reputable mexican pharmacies online mexicopharm.com
indian pharmacies safe: india pharmacy – cheapest online pharmacy india indiapharm.llc
mexican pharmaceuticals online: Purple Pharmacy online ordering – mexican border pharmacies shipping to usa mexicopharm.com
http://mexicopharm.com/# mexican drugstore online mexicopharm.com
https://sildenafildelivery.pro/# sildenafil 100mg uk cheapest
tadalafil tablets 20 mg online Buy tadalafil online tadalafil 20mg lowest price
Buy Levitra 20mg online: Levitra online – Levitra 10 mg buy online
Vardenafil buy online: Vardenafil buy online – Levitra generic best price
https://tadalafildelivery.pro/# tadalafil generic price
Buy generic Levitra online: Buy generic Levitra online – Buy Levitra 20mg online
https://levitradelivery.pro/# Buy generic Levitra online
http://kamagradelivery.pro/# buy kamagra online usa
sildenafil generic no prescription generic sildenafil prescription sildenafil 50 mg online
Vardenafil online prescription: Buy Vardenafil 20mg – Vardenafil price
http://kamagradelivery.pro/# kamagra
tadalafil 20mg lowest price: tadalafil from india – tadalafil 22 mg
http://kamagradelivery.pro/# kamagra
Levitra 20 mg for sale: Levitra best price – Buy Vardenafil online
buy sildenafil mexico
cheap tadalafil tablets: tadalafil generic us – tadalafil daily 5mg
http://tadalafildelivery.pro/# tadalafil tablets
https://levitradelivery.pro/# Levitra online pharmacy
Levitra generic best price: Buy Levitra 20mg online – Levitra 10 mg best price
best ed pills non prescription online ed medications best drug for ed
http://levitradelivery.pro/# buy Levitra over the counter
buy tadalafil india: generic tadalafil from canada – buy generic tadalafil 20mg
sildenafil 20mg generic cost: Buy generic 100mg Sildenafil online – order sildenafil online uk
ed drug prices: ed pills delivery – best treatment for ed
can you buy amoxicillin over the counter in canada: Amoxicillin buy online – where to buy amoxicillin pharmacy
https://stromectol.guru/# ivermectin price
https://amoxil.guru/# buy amoxicillin 500mg online
http://paxlovid.guru/# п»їpaxlovid
paxlovid covid Buy Paxlovid privately Paxlovid over the counter
where can i get cheap clomid: clomid generic – can i purchase cheap clomid without insurance
http://amoxil.guru/# amoxicillin 500 mg online
http://amoxil.guru/# where to get amoxicillin over the counter
http://prednisone.auction/# prednisone 60 mg
paxlovid buy paxlovid price without insurance buy paxlovid online
ivermectin oral solution: stromectol guru – stromectol price us
http://clomid.auction/# buy generic clomid no prescription
http://paxlovid.guru/# paxlovid pill
http://amoxil.guru/# amoxicillin online purchase
https://amoxil.guru/# amoxicillin 500mg capsules
paxlovid buy Buy Paxlovid privately п»їpaxlovid
amoxicillin online canada: buy amoxicillin online with paypal – how to get amoxicillin
http://clomid.auction/# can you get generic clomid prices
http://furosemide.pro/# lasix furosemide 40 mg
lasix 40mg: Buy Lasix No Prescription – buy furosemide online
order generic propecia without insurance: home – get propecia price
buy zithromax online australia buy zithromax over the counter buy generic zithromax online
http://lisinopril.fun/# can i buy lisinopril over the counter in mexico
https://furosemide.pro/# lasix generic name
lasix furosemide: Over The Counter Lasix – buy lasix online
https://finasteride.men/# propecia prices
zestril cost: lisinopril 10 mg tablets price – lisinopril 20 25 mg tab
http://azithromycin.store/# where to buy zithromax in canada
zithromax buy online: Azithromycin 250 buy online – buy zithromax
get propecia without rx: Buy finasteride 1mg – buying cheap propecia online
furosemida 40 mg furosemida lasix tablet
http://lisinopril.fun/# lisinopril 10 mg tablets price
http://furosemide.pro/# lasix for sale
http://finasteride.men/# order propecia no prescription
buy zestoretic: High Blood Pressure – prinivil 5mg tablet
http://furosemide.pro/# lasix tablet
lasix generic: Buy Furosemide – lasix 40mg
lisinopril 20 mg buy: 3 lisinopril – lisinopril 7.5 mg
zithromax cost uk cheapest azithromycin zithromax 500 mg lowest price pharmacy online
furosemide 40mg: Buy Furosemide – generic lasix
https://furosemide.pro/# lasix 100mg
zithromax price canada: buy zithromax z-pak online – buy cheap zithromax online
https://lisinopril.fun/# lisinopril 2 5 mg tablets
http://lisinopril.fun/# lisinopril 10 mg for sale
http://misoprostol.shop/# buy cytotec over the counter
buy cytotec pills online cheap: cheap cytotec – order cytotec online
on line order lisinopril 20mg over the counter lisinopril zestoretic 10 mg
how much is lisinopril 40 mg: High Blood Pressure – zestril 5 mg tablets
http://azithromycin.store/# zithromax cost
purchase lisinopril 40 mg: buy lisinopril canada – lisinopril 5 mg over the counter
https://lisinopril.fun/# lisinopril 80
lisinopril buy without prescription: High Blood Pressure – lisinopril medication prescription
http://furosemide.pro/# furosemide 100 mg
cost generic propecia pill buy cheap propecia pill cost of propecia pills
buying propecia without rx: Buy Finasteride 5mg – buying generic propecia pill
http://misoprostol.shop/# buy cytotec
https://azithromycin.store/# zithromax 250 price
lasix side effects: Buy Lasix No Prescription – furosemide 40 mg
http://lisinopril.fun/# zestril 10mg
comprare farmaci online all’estero: farmacia online piu conveniente – comprare farmaci online con ricetta
pillole per erezione immediata viagra prezzo farmacia viagra online spedizione gratuita
farmacia online senza ricetta: cialis generico – farmacie online sicure
http://kamagraitalia.shop/# farmacia online miglior prezzo
http://sildenafilitalia.men/# miglior sito dove acquistare viagra
https://sildenafilitalia.men/# viagra online spedizione gratuita
top farmacia online: cialis prezzo – farmaci senza ricetta elenco
farmacia online avanafil prezzo п»їfarmacia online migliore
п»їfarmacia online migliore: kamagra gold – farmacie on line spedizione gratuita
https://avanafilitalia.online/# top farmacia online
п»їfarmacia online migliore: kamagra – farmacia online migliore
http://avanafilitalia.online/# comprare farmaci online con ricetta
https://farmaciaitalia.store/# top farmacia online
farmacie online sicure: Cialis senza ricetta – farmacia online senza ricetta
https://tadalafilitalia.pro/# farmacie online affidabili
farmacia online senza ricetta avanafil farmacie online sicure
farmacia online piГ№ conveniente: avanafil generico – comprare farmaci online all’estero
http://tadalafilitalia.pro/# farmacie online affidabili
farmacia online migliore: farmacia online migliore – farmacia online
http://kamagraitalia.shop/# comprare farmaci online all’estero
farmacie online sicure: п»їfarmacia online migliore – acquisto farmaci con ricetta
http://avanafilitalia.online/# farmacie online affidabili
farmaci senza ricetta elenco farmacia online farmacia online
http://farmaciaitalia.store/# top farmacia online
https://kamagraitalia.shop/# farmacie online sicure
acquisto farmaci con ricetta: avanafil prezzo in farmacia – farmacie online sicure
http://farmaciaitalia.store/# farmacia online migliore
farmacia online: farmacia online migliore – farmacie online sicure
https://mexicanpharm.store/# best mexican online pharmacies
medication from mexico pharmacy: mexico pharmacy – buying from online mexican pharmacy
indianpharmacy com п»їlegitimate online pharmacies india best india pharmacy
http://indiapharm.life/# online pharmacy india
canada pharmacy online: cheapest pharmacy canada – canadian pharmacy 24 com
http://indiapharm.life/# buy medicines online in india
best mail order pharmacy canada: pharmacy com canada – canadian pharmacy tampa
pharmacy in canada: best canadian pharmacy to order from – legitimate canadian pharmacy online
http://canadapharm.shop/# canadadrugpharmacy com
canadian family pharmacy: canadapharmacyonline legit – canada pharmacy
https://canadapharm.shop/# best canadian pharmacy online
canadian drug prices: canadian neighbor pharmacy – canada drugs online review
canadian drug pharmacy: canadian pharmacy – canadian drugs online
pet meds without vet prescription canada buy canadian drugs best canadian online pharmacy
http://mexicanpharm.store/# best online pharmacies in mexico
mexico drug stores pharmacies: mexican mail order pharmacies – mexican border pharmacies shipping to usa
https://canadapharm.shop/# canada pharmacy 24h
http://indiapharm.life/# legitimate online pharmacies india
buy prescription drugs from india: best india pharmacy – india pharmacy mail order
canadian drugs pharmacy: canadian online pharmacy – onlinecanadianpharmacy
http://indiapharm.life/# best online pharmacy india
top online pharmacy india: buy medicines online in india – pharmacy website india
top 10 online pharmacy in india: best india pharmacy – indian pharmacy
https://mexicanpharm.store/# mexico drug stores pharmacies
buy prescription drugs from india: indian pharmacy online – top online pharmacy india
Online medicine home delivery п»їlegitimate online pharmacies india indian pharmacy paypal
http://canadapharm.shop/# reliable canadian pharmacy
https://indiapharm.life/# india online pharmacy
pharmacies in mexico that ship to usa: medication from mexico pharmacy – mexican online pharmacies prescription drugs
legitimate canadian pharmacy: best mail order pharmacy canada – ordering drugs from canada
https://canadapharm.shop/# canada drugs online
medication from mexico pharmacy: reputable mexican pharmacies online – mexico drug stores pharmacies
canadian pharmacy checker: canadian online pharmacy reviews – www canadianonlinepharmacy
http://mexicanpharm.store/# mexico pharmacies prescription drugs
world pharmacy india: indian pharmacy paypal – pharmacy website india
http://mexicanpharm.store/# mexican drugstore online
https://canadapharm.shop/# 77 canadian pharmacy
canada discount pharmacy canadian pharmacy online canadian pharmacies
mexico drug stores pharmacies: pharmacies in mexico that ship to usa – best online pharmacies in mexico
http://mexicanpharm.store/# buying prescription drugs in mexico
rate canadian pharmacies: canadian pharmacies that deliver to the us – safe canadian pharmacy
canadian 24 hour pharmacy: best canadian pharmacy to order from – certified canadian pharmacy
prednisone 40 mg daily: prednisone 10 mg online – prednisone pills 10 mg
https://nolvadex.pro/# femara vs tamoxifen
Unrivaled in the sphere of international pharmacy http://cytotec.directory/# buy misoprostol over the counter
buy cytotec pills cytotec pills buy online purchase cytotec
purchase cytotec: Abortion pills online – cytotec buy online usa
Always on the pulse of international healthcare developments http://prednisonepharm.store/# prednisone pak
https://zithromaxpharm.online/# generic zithromax azithromycin
http://zithromaxpharm.online/# zithromax capsules australia
Always stocked with what I need http://clomidpharm.shop/# can i get clomid price
http://zithromaxpharm.online/# zithromax cost canada
can i buy prednisone from canada without a script: buying prednisone from canada – otc prednisone cream
Their commitment to healthcare excellence is evident https://cytotec.directory/# buy cytotec over the counter
http://clomidpharm.shop/# where can i buy cheap clomid without a prescription
order cytotec online: buy cytotec over the counter – buy cytotec pills online cheap
how to get cheap clomid prices clomid online where can i get clomid without rx
They make international medication sourcing a breeze http://prednisonepharm.store/# prednisone cost us
https://prednisonepharm.store/# prednisone 10mg
https://prednisonepharm.store/# prednisone 4 mg daily
tamoxifen buy: how does tamoxifen work – alternatives to tamoxifen
They provide global solutions to local health challenges http://nolvadex.pro/# tamoxifen side effects forum
https://clomidpharm.shop/# how to buy generic clomid without rx
Their worldwide pharmacists’ consultations are invaluable https://prednisonepharm.store/# prednisone acetate
prednisone online sale: prednisone price australia – prednisone 5 mg tablet cost
https://nolvadex.pro/# tamoxifen endometriosis
where buy generic clomid pill where can i buy generic clomid now order cheap clomid without a prescription
A trusted partner for patients worldwide https://clomidpharm.shop/# cheap clomid now
zithromax online: cheap zithromax pills – zithromax 250 price
http://cytotec.directory/# Cytotec 200mcg price
Their global health initiatives are game-changers https://nolvadex.pro/# nolvadex for pct
http://reputablepharmacies.online/# pharmacy cost comparison
best rated canadian online pharmacy https://edpills.bid/# non prescription ed drugs
pharmacy drug store online no rx
non prescription ed drugs buy prescription drugs online cialis without a doctor’s prescription
online meds without presxription: online pharmacies canada – on line pharmacy with no perscriptions
https://reputablepharmacies.online/# best price prescription drugs
prescription drugs online without ed prescription drugs meds online without doctor prescription
best treatment for ed: medications for ed – the best ed pill
my mexican drugstore: legitimate mexican pharmacy online – canadain pharmacy no prescription
https://reputablepharmacies.online/# canada pharmacies without script
online canadian pharcharmy canada rx pharmacy online drugstore service canada
https://edwithoutdoctorprescription.store/# prescription drugs online
non prescription ed pills: sildenafil without a doctor’s prescription – real cialis without a doctor’s prescription
certified mexican pharmacy http://reputablepharmacies.online/# canadian pharmacy without prescription
top 10 online pharmacies
ed medications online online ed pills new ed treatments
http://edpills.bid/# generic ed drugs
list of canadian pharmacies online: best mail order pharmacies – canadian rx
generic ed drugs top ed drugs the best ed pills
ed meds online without doctor prescription: how to get prescription drugs without doctor – prescription drugs without doctor approval
http://edwithoutdoctorprescription.store/# buy prescription drugs online legally
compare ed drugs: treatment for ed – best treatment for ed
best male enhancement pills best ed pills at gnc erectile dysfunction drugs
http://edpills.bid/# gnc ed pills
prescription without a doctor’s prescription prescription cost comparison pharmacy online canada
prescription drugs online without doctor: buy prescription drugs from canada – online prescription for ed meds
https://reputablepharmacies.online/# online prescriptions canada without
canadian pharmacy online no prescription my canadian family pharmacy canadian drugs
ed medication: new treatments for ed – erectile dysfunction pills
https://edpills.bid/# over the counter erectile dysfunction pills
prescription drugs without doctor approval non prescription erection pills buy prescription drugs from india
best drug for ed: online ed medications – best erection pills
prescription drugs canada buy online: viagra without a doctor prescription – non prescription ed drugs
http://mexicanpharmacy.win/# reputable mexican pharmacies online mexicanpharmacy.win
https://indianpharmacy.shop/# reputable indian pharmacies indianpharmacy.shop
canadian pharmacy 24hr
canadian pharmacy phone number Pharmacies in Canada that ship to the US reputable canadian online pharmacies canadianpharmacy.pro
mexico pharmacy: Medicines Mexico – best online pharmacies in mexico mexicanpharmacy.win
http://canadianpharmacy.pro/# canadapharmacyonline legit canadianpharmacy.pro
Online medicine order п»їlegitimate online pharmacies india best india pharmacy indianpharmacy.shop
northwest pharmacy canada: Pharmacies in Canada that ship to the US – online canadian pharmacy review canadianpharmacy.pro
india pharmacy indian pharmacy to usa indian pharmacy indianpharmacy.shop
http://canadianpharmacy.pro/# canadian pharmacy com canadianpharmacy.pro
mexico pharmacy: Mexico pharmacy – mexican drugstore online mexicanpharmacy.win
http://canadianpharmacy.pro/# canadian valley pharmacy canadianpharmacy.pro
canada pharmacy online no script
http://indianpharmacy.shop/# Online medicine order indianpharmacy.shop
mexican pharmaceuticals online mexican pharmacy online mexican rx online mexicanpharmacy.win
https://canadianpharmacy.pro/# canadian pharmacy online ship to usa canadianpharmacy.pro
mail order pharmacy india Cheapest online pharmacy buy prescription drugs from india indianpharmacy.shop
https://mexicanpharmacy.win/# mexican mail order pharmacies mexicanpharmacy.win
medication from mexico pharmacy Mexico pharmacy buying prescription drugs in mexico online mexicanpharmacy.win
best canadian online pharmacy Canada Pharmacy canadian pharmacy world canadianpharmacy.pro
https://canadianpharmacy.pro/# best mail order pharmacy canada canadianpharmacy.pro
http://indianpharmacy.shop/# top 10 online pharmacy in india indianpharmacy.shop
indian pharmacy online
https://canadianpharmacy.pro/# canadian drug pharmacy canadianpharmacy.pro
http://canadianpharmacy.pro/# canadian medications canadianpharmacy.pro
canadian pharmacies review
mexican pharmaceuticals online Mexico pharmacy mexican border pharmacies shipping to usa mexicanpharmacy.win
https://mexicanpharmacy.win/# medicine in mexico pharmacies mexicanpharmacy.win
mail order pharmacy india
http://canadianpharmacy.pro/# canadian pharmacy 24h com canadianpharmacy.pro
mexico pharmacies prescription drugs medication from mexico pharmacy mexican online pharmacies prescription drugs mexicanpharmacy.win
https://mexicanpharmacy.win/# mexico drug stores pharmacies mexicanpharmacy.win
п»їlegitimate online pharmacies india
https://mexicanpharmacy.win/# purple pharmacy mexico price list mexicanpharmacy.win
http://canadianpharmacy.pro/# canadianpharmacymeds com canadianpharmacy.pro
indianpharmacy com
canadianpharmacyworld northwest pharmacy canada trusted canadian pharmacy canadianpharmacy.pro
https://mexicanpharmacy.win/# mexico pharmacy mexicanpharmacy.win
http://mexicanpharmacy.win/# mexican online pharmacies prescription drugs mexicanpharmacy.win
best india pharmacy
indian pharmacy international medicine delivery from india india online pharmacy indianpharmacy.shop
https://indianpharmacy.shop/# top 10 pharmacies in india indianpharmacy.shop
indian pharmacy online
best india pharmacy international medicine delivery from india pharmacy website india indianpharmacy.shop
https://canadianpharmacy.pro/# canadian pharmacy 365 canadianpharmacy.pro
https://indianpharmacy.shop/# best india pharmacy indianpharmacy.shop
Pharmacie en ligne sans ordonnance levitra generique Pharmacie en ligne pas cher
Viagra gГ©nГ©rique pas cher livraison rapide: Viagra sans ordonnance 24h – Viagra pas cher livraison rapide france
https://cialissansordonnance.shop/# acheter medicament a l etranger sans ordonnance
https://pharmadoc.pro/# Pharmacie en ligne sans ordonnance
pharmacie ouverte
Pharmacie en ligne livraison gratuite: Levitra acheter – Acheter mГ©dicaments sans ordonnance sur internet
Viagra homme prix en pharmacie sans ordonnance: Viagra Pfizer sans ordonnance – Viagra sans ordonnance 24h
https://viagrasansordonnance.pro/# Viagra pas cher livraison rapide france
Pharmacie en ligne sans ordonnance Levitra pharmacie en ligne Pharmacie en ligne pas cher
http://pharmadoc.pro/# Pharmacie en ligne livraison rapide
Pharmacie en ligne pas cher pharmacie ouverte 24/24 pharmacie ouverte
Pharmacie en ligne livraison rapide: pharmacie en ligne pas cher – pharmacie ouverte
https://levitrasansordonnance.pro/# Pharmacie en ligne livraison 24h
Acheter mГ©dicaments sans ordonnance sur internet
https://acheterkamagra.pro/# Pharmacies en ligne certifiées
Pharmacie en ligne fiable: Acheter Cialis – Pharmacie en ligne pas cher
Pharmacie en ligne pas cher: Cialis sans ordonnance 24h – Pharmacie en ligne France
https://acheterkamagra.pro/# Pharmacie en ligne sans ordonnance
Viagra pas cher inde viagrasansordonnance.pro SildГ©nafil 100mg pharmacie en ligne
Pharmacie en ligne fiable: Levitra 20mg prix en pharmacie – Pharmacies en ligne certifiГ©es
Pharmacie en ligne fiable Levitra sans ordonnance 24h pharmacie ouverte 24/24
pharmacie ouverte 24/24: Levitra acheter – acheter medicament a l etranger sans ordonnance
prednisone 50 mg price prednisone prices prednisone 20
ivermectin 400 mg brands: can you buy stromectol over the counter – buy stromectol online
https://ivermectin.store/# ivermectin 1mg
https://amoxicillin.bid/# amoxicillin azithromycin
prednisone 20mg online pharmacy prednisone 50 mg prices 20 mg of prednisone
stromectol ivermectin tablets: generic stromectol – ivermectin usa
stromectol price us: minocycline acne – minocin 50 mg for scabies
https://ivermectin.store/# ivermectin goodrx
order generic clomid for sale: can i get clomid pills – can you buy cheap clomid pill
stromectol 3 mg tablet ivermectin 4 mg stromectol tablets for humans for sale
https://ivermectin.store/# stromectol generic name
buy prednisone with paypal canada: 50mg prednisone tablet – where can i buy prednisone
ivermectin 400 mg ivermectin buy where can i buy oral ivermectin
https://amoxicillin.bid/# amoxicillin generic brand
where can i buy amoxicillin over the counter uk: cost of amoxicillin prescription – amoxicillin 250 mg price in india
https://ivermectin.store/# ivermectin generic cream
buy amoxicillin: amoxicillin 500 mg tablet price – amoxicillin 500 mg capsule
https://ivermectin.store/# buy ivermectin for humans australia
can i get cheap clomid without rx where can i get generic clomid without a prescription can i buy cheap clomid online
can i order generic clomid without rx: where to get cheap clomid for sale – where to buy cheap clomid prices
http://amoxicillin.bid/# medicine amoxicillin 500mg
zithromax price south africa zithromax price canada zithromax prescription
azithromycin zithromax: average cost of generic zithromax – zithromax for sale usa
generic zithromax india: zithromax 500 price – zithromax for sale usa
https://prednisonetablets.shop/# prednisone 20mg price
can you buy prednisone without a prescription: prednisone for sale no prescription – prednisone buy no prescription
http://amoxicillin.bid/# amoxicillin 500 mg for sale
zithromax capsules buy zithromax online cheap zithromax 1000 mg online
http://amoxicillin.bid/# where to buy amoxicillin over the counter
where can i buy oral ivermectin: ivermectin 1mg – ivermectin pills human
canadian pharmacy meds canadian pharmacies online global pharmacy canada canadianpharm.store
world pharmacy india: order medicine from india to usa – indian pharmacy online indianpharm.store
https://mexicanpharm.shop/# mexican drugstore online mexicanpharm.shop
buying prescription drugs in mexico: mexico drug stores pharmacies – mexican pharmaceuticals online mexicanpharm.shop
purple pharmacy mexico price list: buying from online mexican pharmacy – mexican drugstore online mexicanpharm.shop
http://mexicanpharm.shop/# buying prescription drugs in mexico online mexicanpharm.shop
https://indianpharm.store/# Online medicine home delivery indianpharm.store
top 10 pharmacies in india: buy prescription drugs from india – best india pharmacy indianpharm.store
mexican rx online Online Mexican pharmacy mexico drug stores pharmacies mexicanpharm.shop
online canadian pharmacy: Licensed Online Pharmacy – safe online pharmacies in canada canadianpharm.store
https://canadianpharm.store/# canadian pharmacy 24h com safe canadianpharm.store
canadianpharmacyworld com: Licensed Online Pharmacy – canadian pharmacy sarasota canadianpharm.store
india pharmacy mail order: order medicine from india to usa – indian pharmacy paypal indianpharm.store
mexican rx online Online Pharmacies in Mexico п»їbest mexican online pharmacies mexicanpharm.shop
http://canadianpharm.store/# canadian pharmacy world canadianpharm.store
buying from online mexican pharmacy: Online Pharmacies in Mexico – purple pharmacy mexico price list mexicanpharm.shop
https://indianpharm.store/# top 10 pharmacies in india indianpharm.store
online canadian drugstore Certified Online Pharmacy Canada canadianpharmacymeds com canadianpharm.store
online pharmacy india: order medicine from india to usa – online pharmacy india indianpharm.store
best online pharmacy india: mail order pharmacy india – india pharmacy mail order indianpharm.store
http://indianpharm.store/# india pharmacy mail order indianpharm.store
https://indianpharm.store/# Online medicine order indianpharm.store
top 10 pharmacies in india: order medicine from india to usa – indian pharmacy paypal indianpharm.store
canadian pharmacy: Canada Pharmacy online – canadian pharmacies online canadianpharm.store
buying from online mexican pharmacy Online Mexican pharmacy mexican rx online mexicanpharm.shop
https://indianpharm.store/# top 10 pharmacies in india indianpharm.store
ed meds online canada: Pharmacies in Canada that ship to the US – pharmacy rx world canada canadianpharm.store
canadian pharmacy online canada drugs online review canadian pharmacy ltd canadianpharm.store
https://indianpharm.store/# indian pharmacy paypal indianpharm.store
canadian pharmacy online ship to usa: Canadian International Pharmacy – best canadian online pharmacy canadianpharm.store
buy prescription drugs from india: order medicine from india to usa – india pharmacy mail order indianpharm.store
https://indianpharm.store/# indian pharmacy indianpharm.store
http://mexicanpharm.shop/# mexico drug stores pharmacies mexicanpharm.shop
mexican pharmacy: Online Mexican pharmacy – mexican online pharmacies prescription drugs mexicanpharm.shop
canadian world pharmacy Best Canadian online pharmacy canadian drug canadianpharm.store
indian pharmacy online: reputable indian online pharmacy – india online pharmacy indianpharm.store
https://mexicanpharm.shop/# mexico drug stores pharmacies mexicanpharm.shop
canadian compounding pharmacy: buying drugs from canada – canadian pharmacies comparison canadianpharm.store
mexican pharmaceuticals online Online Pharmacies in Mexico п»їbest mexican online pharmacies mexicanpharm.shop
canadian pharmacy online ship to usa: Canada Pharmacy online – maple leaf pharmacy in canada canadianpharm.store
https://mexicanpharm.shop/# mexican mail order pharmacies mexicanpharm.shop
top 10 pharmacies in india: Indian pharmacy to USA – Online medicine order indianpharm.store
canadian discount pharmacy Canadian Pharmacy canada drugs online reviews canadianpharm.store
http://indianpharm.store/# buy medicines online in india indianpharm.store
buy medicines online in india: order medicine from india to usa – indian pharmacy indianpharm.store
http://indianpharm.store/# best india pharmacy indianpharm.store
reputable mexican pharmacies online: mexican pharmaceuticals online – pharmacies in mexico that ship to usa mexicanpharm.shop
buying drugs canada legal canadian prescription drugs online canadian prescription pharmacy
canadian pharmacy certified: most reliable canadian pharmacies – legitimate canadian pharmacies
https://canadadrugs.pro/# us online pharmacy
mexican pharmacy drugs: nabp approved canadian pharmacies – canadian drug stores
canada pharmacies: best online pharmacies reviews – canada pharmacy reviews
ed meds online my canadian pharmacy rx reviews meds without prescription
prescription price checker: ed meds online – bestpharmacyonline.com
https://canadadrugs.pro/# canadian pharmacy drug prices
overseas pharmacies canada pharmacy not requiring prescription canada prescription
online prescriptions without a doctor: trusted online canadian pharmacy – top mail order pharmacies
certified canadian pharmacies: cheap canadian cialis – rx prices
http://canadadrugs.pro/# aarp approved canadian pharmacies
certified canadian international pharmacy: best mexican online pharmacies – online pharmacy reviews
canadian pharmaceutical companies that ship to usa doxycycline mexican pharmacy no prescription rx medicine
https://canadadrugs.pro/# online pharmacy review
safe canadian pharmacy: online meds without presxription – recommended canadian online pharmacies
canadian online pharmacies: canadian pharmacy antiobotics without perscription – canadian pharmacy delivery
http://canadadrugs.pro/# giant discount pharmacy
canadian prescription drug prices: canadian online pharmacies – canadian drug store online
canadadrugpharmacy: canadian drug store prices – no prescription rx medicine
https://canadadrugs.pro/# online pharmacy without a prescription
canada pharmacies online: mexican drugstore online – aarp canadian pharmacy
https://canadadrugs.pro/# canadian pharmacy prices
https://canadadrugs.pro/# certified canadian pharmacy
trust online pharmacies: rx online no prior prescription – list of mexican pharmacies
approved canadian pharmacies online: buying drugs canada – no script pharmacy
meds without prescription: canadian pharmacy cialis cheap – canadian pharmacy products
canadian pharmacies selling cialis: safe online pharmacies – best canadian prescription prices
https://canadadrugs.pro/# no prescription rx medicine
http://canadadrugs.pro/# on line pharmacy with no prescriptions
certified online canadian pharmacies: canadian prescription pharmacy – mail order prescription drugs
canadian pharmacy sarasota canadian pharmacy store canadian king pharmacy
best ed drug: п»їerectile dysfunction medication – ed meds online
http://medicinefromindia.store/# reputable indian pharmacies
ed medications online: top ed pills – ed drugs compared
online pharmacy india п»їlegitimate online pharmacies india indian pharmacy
http://edpill.cheap/# what is the best ed pill
prescription drugs online without doctor: generic cialis without a doctor prescription – non prescription erection pills
http://certifiedpharmacymexico.pro/# best mexican online pharmacies
best over the counter ed pills best ed pills at gnc erectile dysfunction medications
https://medicinefromindia.store/# Online medicine home delivery
buy prescription drugs from canada cheap cheap cialis ed meds online without doctor prescription
buy ed pills online: ed pills that really work – best ed pills non prescription
https://edwithoutdoctorprescription.pro/# how to get prescription drugs without doctor
buying drugs from canada: canadian pharmacy tampa – canada drugs reviews
п»їerectile dysfunction medication ed pills gnc gnc ed pills
https://edwithoutdoctorprescription.pro/# best non prescription ed pills
https://edpill.cheap/# ed meds
buy prescription drugs online cialis without a doctor prescription canada prescription drugs without doctor approval
mexican mail order pharmacies: п»їbest mexican online pharmacies – mexico pharmacy
http://canadianinternationalpharmacy.pro/# canadian pharmacies online
medication from mexico pharmacy: medicine in mexico pharmacies – best online pharmacies in mexico
canada drug pharmacy canadian online pharmacy reviews online canadian pharmacy
https://medicinefromindia.store/# best india pharmacy
viagra without a doctor prescription buy prescription drugs from canada buy cheap prescription drugs online
http://certifiedpharmacymexico.pro/# mexican mail order pharmacies
http://edpill.cheap/# male ed pills
mexican pharmaceuticals online buying from online mexican pharmacy pharmacies in mexico that ship to usa
pet meds without vet prescription canada: escrow pharmacy canada – 77 canadian pharmacy
http://medicinefromindia.store/# best india pharmacy
Online medicine order mail order pharmacy india india online pharmacy
http://edwithoutdoctorprescription.pro/# prescription drugs without doctor approval
medication from mexico pharmacy mexican online pharmacies prescription drugs п»їbest mexican online pharmacies
https://certifiedpharmacymexico.pro/# mexico drug stores pharmacies
best pills for ed buying ed pills online cheap erectile dysfunction pills online
ed meds online without doctor prescription: cialis without a doctor prescription canada – discount prescription drugs
https://canadianinternationalpharmacy.pro/# the canadian drugstore
buy prescription drugs from india reputable indian online pharmacy п»їlegitimate online pharmacies india
http://medicinefromindia.store/# world pharmacy india
http://canadianinternationalpharmacy.pro/# reputable canadian online pharmacies
online canadian pharmacy reviews best rated canadian pharmacy onlinecanadianpharmacy 24
https://edwithoutdoctorprescription.pro/# non prescription ed pills
mexico drug stores pharmacies: п»їbest mexican online pharmacies – medication from mexico pharmacy
erection pills online cheap ed pills best ed drug
https://edwithoutdoctorprescription.pro/# prescription drugs
ed remedies mens ed pills best ed treatment pills
https://medicinefromindia.store/# legitimate online pharmacies india
https://medicinefromindia.store/# buy medicines online in india
top 10 online pharmacy in india indian pharmacy paypal india pharmacy mail order
https://medicinefromindia.store/# Online medicine home delivery
prescription drugs canada buy online: canadian pharmacy online store – canada pharmacy world
online shopping pharmacy india п»їlegitimate online pharmacies india pharmacy website india
http://edpill.cheap/# impotence pills
medication from mexico pharmacy mexico drug stores pharmacies buying from online mexican pharmacy
medication from mexico pharmacy mexican rx online mexican pharmacy
http://mexicanph.shop/# mexican mail order pharmacies
medication from mexico pharmacy
best online pharmacies in mexico pharmacies in mexico that ship to usa mexican mail order pharmacies
mexican pharmaceuticals online mexican border pharmacies shipping to usa mexican rx online
medication from mexico pharmacy mexican pharmaceuticals online buying prescription drugs in mexico
mexico pharmacies prescription drugs mexico pharmacies prescription drugs buying from online mexican pharmacy
buying from online mexican pharmacy mexican pharmacy medicine in mexico pharmacies
best online pharmacies in mexico mexico drug stores pharmacies buying from online mexican pharmacy
mexico pharmacy mexico pharmacies prescription drugs mexico drug stores pharmacies
http://mexicanph.shop/# purple pharmacy mexico price list
buying prescription drugs in mexico online
mexican rx online buying from online mexican pharmacy buying prescription drugs in mexico online
medicine in mexico pharmacies mexico pharmacy buying prescription drugs in mexico
mexican pharmacy mexican pharmacy mexican mail order pharmacies
buying prescription drugs in mexico online best mexican online pharmacies medicine in mexico pharmacies
buying from online mexican pharmacy buying prescription drugs in mexico online п»їbest mexican online pharmacies
mexico drug stores pharmacies mexican drugstore online pharmacies in mexico that ship to usa
mexican pharmacy best online pharmacies in mexico medicine in mexico pharmacies
buying prescription drugs in mexico mexican drugstore online medication from mexico pharmacy
mexican rx online mexico drug stores pharmacies mexican mail order pharmacies
mexican pharmacy mexican pharmacy medication from mexico pharmacy
buying prescription drugs in mexico online medication from mexico pharmacy п»їbest mexican online pharmacies
mexico pharmacies prescription drugs mexican pharmacy buying prescription drugs in mexico
https://mexicanph.shop/# mexican pharmaceuticals online
mexican drugstore online
mexican rx online mexican drugstore online reputable mexican pharmacies online
п»їbest mexican online pharmacies buying prescription drugs in mexico online pharmacies in mexico that ship to usa
mexican online pharmacies prescription drugs mexico pharmacy mexico drug stores pharmacies
reputable mexican pharmacies online mexican pharmaceuticals online mexican rx online
mexican pharmacy mexico pharmacies prescription drugs mexican pharmacy
buying from online mexican pharmacy mexican rx online medicine in mexico pharmacies
mexican pharmaceuticals online purple pharmacy mexico price list mexican online pharmacies prescription drugs
best mexican online pharmacies mexican pharmacy pharmacies in mexico that ship to usa
mexican pharmacy mexico drug stores pharmacies pharmacies in mexico that ship to usa
https://mexicanph.com/# buying prescription drugs in mexico
mexico drug stores pharmacies
best online pharmacies in mexico reputable mexican pharmacies online buying prescription drugs in mexico
mexico drug stores pharmacies mexico drug stores pharmacies mexican mail order pharmacies
mexican border pharmacies shipping to usa mexico drug stores pharmacies mexico drug stores pharmacies
mexican mail order pharmacies pharmacies in mexico that ship to usa medication from mexico pharmacy
mexico drug stores pharmacies medicine in mexico pharmacies purple pharmacy mexico price list
mexican drugstore online mexican rx online mexican border pharmacies shipping to usa
medication from mexico pharmacy mexican pharmaceuticals online medicine in mexico pharmacies
best mexican online pharmacies mexico pharmacy buying prescription drugs in mexico online
mexico pharmacy best online pharmacies in mexico mexican drugstore online
mexican mail order pharmacies mexico drug stores pharmacies buying prescription drugs in mexico
http://mexicanph.com/# buying from online mexican pharmacy
medicine in mexico pharmacies
mexican mail order pharmacies mexican pharmacy п»їbest mexican online pharmacies
reputable mexican pharmacies online mexico pharmacy mexican pharmaceuticals online
mexican pharmacy mexican border pharmacies shipping to usa reputable mexican pharmacies online
mexican mail order pharmacies mexican mail order pharmacies medicine in mexico pharmacies
reputable mexican pharmacies online mexican pharmaceuticals online purple pharmacy mexico price list
mexico drug stores pharmacies buying prescription drugs in mexico online pharmacies in mexico that ship to usa
mexican online pharmacies prescription drugs mexican rx online mexican pharmacy
mexico drug stores pharmacies pharmacies in mexico that ship to usa mexican mail order pharmacies
mexican border pharmacies shipping to usa buying prescription drugs in mexico online mexico pharmacy
buying from online mexican pharmacy mexican mail order pharmacies best online pharmacies in mexico
purple pharmacy mexico price list buying prescription drugs in mexico online mexican drugstore online
best online pharmacies in mexico mexico pharmacies prescription drugs mexico pharmacies prescription drugs
http://mexicanph.shop/# mexico pharmacies prescription drugs
medication from mexico pharmacy
best mexican online pharmacies buying from online mexican pharmacy best mexican online pharmacies
buying prescription drugs in mexico online mexican rx online mexico pharmacies prescription drugs
mexico pharmacies prescription drugs mexican drugstore online medicine in mexico pharmacies
mexico pharmacies prescription drugs mexican drugstore online mexico drug stores pharmacies
mexican pharmacy buying from online mexican pharmacy mexico pharmacies prescription drugs
mexico pharmacies prescription drugs mexico drug stores pharmacies mexican mail order pharmacies
mexican rx online pharmacies in mexico that ship to usa medication from mexico pharmacy
best mexican online pharmacies mexican border pharmacies shipping to usa medicine in mexico pharmacies
pharmacies in mexico that ship to usa mexican drugstore online medication from mexico pharmacy
pharmacies in mexico that ship to usa medicine in mexico pharmacies buying from online mexican pharmacy
mexican mail order pharmacies reputable mexican pharmacies online mexican rx online
https://mexicanph.com/# reputable mexican pharmacies online
buying prescription drugs in mexico online
mexican pharmacy buying from online mexican pharmacy buying prescription drugs in mexico
mexican drugstore online buying prescription drugs in mexico purple pharmacy mexico price list
mexican pharmaceuticals online pharmacies in mexico that ship to usa purple pharmacy mexico price list
purple pharmacy mexico price list mexican drugstore online mexican border pharmacies shipping to usa
mexican online pharmacies prescription drugs mexico drug stores pharmacies mexican pharmaceuticals online
mexican pharmacy mexico drug stores pharmacies best online pharmacies in mexico
buying prescription drugs in mexico online mexican pharmacy mexico drug stores pharmacies
mexico drug stores pharmacies mexican pharmaceuticals online mexico pharmacy
best online pharmacies in mexico mexican online pharmacies prescription drugs mexican border pharmacies shipping to usa
http://stromectol.fun/# ivermectin uk coronavirus
furosemide 100mg: Buy Furosemide – lasix 20 mg
buy amoxicillin from canada: amoxicillin 500 mg tablet price – amoxicillin 825 mg
https://amoxil.cheap/# purchase amoxicillin 500 mg
amoxicillin 875 mg tablet buy amoxicillin online cheap where can you get amoxicillin
http://buyprednisone.store/# can i buy prednisone from canada without a script
furosemide 40 mg: Buy Lasix – lasix furosemide
https://amoxil.cheap/# amoxicillin 500mg capsule cost
amoxicillin 750 mg price: how to get amoxicillin over the counter – how much is amoxicillin prescription
stromectol generic name generic name for ivermectin stromectol where to buy
stromectol price usa: ivermectin australia – ivermectin cream cost
https://lisinopril.top/# prinivil brand name
http://buyprednisone.store/# price for 15 prednisone
ivermectin 4 mg ivermectin gel stromectol pill
amoxicillin discount: buy amoxicillin online cheap – amoxicillin tablets in india
https://stromectol.fun/# ivermectin lotion price
prednisone online sale: 40 mg daily prednisone – prednisone canada prices
http://lisinopril.top/# lisinopril 419
lasix uses: buy furosemide online – lasix tablet
ivermectin 0.08 stromectol medicine stromectol tablets for humans
https://stromectol.fun/# minocycline 100mg for acne
https://furosemide.guru/# furosemide 100mg
lasix tablet: lasix 100mg – lasix 100 mg tablet
ivermectin 3mg: stromectol uk buy – stromectol south africa
http://lisinopril.top/# lisinopril 10 mg price in india
lisinopril 20 mg uk buy prinivil zestril 10 mg
prednisone 5 mg: 1250 mg prednisone – prednisone
https://stromectol.fun/# where to buy ivermectin pills
lasix for sale: Buy Furosemide – lasix 40 mg
furosemida: Buy Lasix – lasix 40mg
https://furosemide.guru/# lasix online
lasix 20 mg Buy Lasix furosemide 100 mg
http://furosemide.guru/# generic lasix
prednisone 30 mg tablet: prednisone cost us – buying prednisone
http://stromectol.fun/# minocycline medication
over the counter amoxicillin canada: amoxicillin generic – amoxicillin 875 mg tablet
https://amoxil.cheap/# amoxicillin no prescription
lisinopril 20 tablet lisinopril 40 mg brand name in india zestril tablet price
buy prednisone online from canada: prednisone 60 mg tablet – 5 mg prednisone daily
http://buyprednisone.store/# prednisone 20 mg without prescription
stromectol ivermectin 3 mg: generic ivermectin – ivermectin price
https://stromectol.fun/# ivermectin pills human
ivermectin human: ivermectin 0.5% lotion – ivermectin 8000
stromectol tab 3mg generic ivermectin stromectol lotion
side effects of lisinopril 10mg
ivermectin 0.08 oral solution: stromectol in canada – ivermectin 50
http://stromectol.fun/# stromectol tablets for humans for sale
http://buyprednisone.store/# no prescription prednisone canadian pharmacy
http://furosemide.guru/# lasix furosemide 40 mg
zestril 2.5: lisinopril 5mg tabs – lisinopril 2 mg
lasix for sale furosemide 40mg lasix furosemide 40 mg
buy prednisone online without a prescription: prednisone over the counter – prednisone 3 tablets daily
http://furosemide.guru/# furosemide
buy amoxicillin canada: amoxicillin 250 mg capsule – amoxicillin 500mg over the counter
lasix 40 mg furosemide 40 mg lasix dosage
ivermectin cream uk: how to buy stromectol – ivermectin tablet 1mg
lasix 40 mg: Buy Lasix – lasix furosemide
https://lisinopril.top/# zestril cost price
http://lisinopril.top/# lisinopril 10 mg online no prescription
amoxicillin 500mg prescription: amoxicillin from canada – amoxil pharmacy
http://stromectol.fun/# ivermectin buy uk
prednisone 60 mg daily where to buy prednisone 20mg no prescription prednisone 20mg cheap
http://amoxil.cheap/# where can i get amoxicillin 500 mg
lasix generic: lasix generic – lasix 40 mg
1250 mg prednisone: prednisone canada pharmacy – prednisone 5 mg cheapest
http://stromectol.fun/# ivermectin cream uk
generic amoxil 500 mg can you purchase amoxicillin online amoxicillin 500mg price in canada
prednisone 10mg canada: prednisone 30 mg coupon – prednisone brand name
https://amoxil.cheap/# generic amoxicillin 500mg
prednisone canada prescription: order prednisone from canada – where to buy prednisone in canada
https://stromectol.fun/# ivermectin drug
where can you get amoxicillin: amoxicillin medicine over the counter – amoxicillin 500mg capsules antibiotic
lasix generic name Buy Lasix No Prescription furosemide 100mg
http://furosemide.guru/# lasix side effects
http://buyprednisone.store/# prednisone in india
can i buy lisinopril over the counter: zestril cost – cost of brand name lisinopril
https://amoxil.cheap/# amoxicillin discount coupon
ivermectin 20 mg: ivermectin 3mg tablet – ivermectin 3mg dose
http://stromectol.fun/# stromectol sales
discount zestril lisinopril 19 mg zestoretic 10 mg
india buy prednisone online: cost of prednisone – generic prednisone otc
https://amoxil.cheap/# buy amoxicillin canada
lasix online: Buy Lasix No Prescription – buy lasix online
buy amoxicillin online with paypal: amoxicillin without prescription – amoxicillin cephalexin
http://stromectol.fun/# ivermectin 0.5% brand name
amoxicillin for sale online amoxicillin buy online canada purchase amoxicillin online
https://lisinopril.top/# where to buy lisinopril without prescription
https://lisinopril.top/# zestoretic coupon
can you purchase amoxicillin online: over the counter amoxicillin canada – purchase amoxicillin online without prescription
https://furosemide.guru/# lasix for sale
indian pharmacy buy prescription drugs from india india pharmacy mail order
indianpharmacy com pharmacy website india indian pharmacy
https://indianph.xyz/# indian pharmacies safe
india online pharmacy
pharmacy website india indian pharmacies safe top online pharmacy india
reputable indian online pharmacy indianpharmacy com india online pharmacy
https://indianph.xyz/# indian pharmacy paypal
Online medicine home delivery
https://indianph.xyz/# best india pharmacy
best online pharmacy india
п»їlegitimate online pharmacies india india pharmacy indianpharmacy com
http://indianph.xyz/# Online medicine order
http://indianph.com/# india online pharmacy
indian pharmacy
https://indianph.xyz/# pharmacy website india
pharmacy website india
https://indianph.com/# indianpharmacy com
best india pharmacy
india pharmacy mail order indianpharmacy com cheapest online pharmacy india
cephalexin online
http://indianph.xyz/# top 10 pharmacies in india
best india pharmacy
escitalopram oxalate vs escitalopram
best online pharmacy india Online medicine home delivery buy medicines online in india
http://indianph.com/# Online medicine home delivery
top 10 pharmacies in india
http://cytotec24.com/# п»їcytotec pills online
buy ciprofloxacin: buy ciprofloxacin over the counter – ciprofloxacin 500mg buy online
cost of tamoxifen is nolvadex legal tamoxifen medication
http://diflucan.pro/# can you buy diflucan over the counter
buy cheap doxycycline online: how to buy doxycycline online – doxycycline 100mg
http://nolvadex.guru/# tamoxifen and depression
buy cytotec online buy cytotec online buy cytotec in usa
ciprofloxacin generic price: cipro pharmacy – buy cipro cheap
http://cipro.guru/# ciprofloxacin 500mg buy online
http://doxycycline.auction/# purchase doxycycline online
http://cytotec24.shop/# cytotec pills online
diflucan cream over the counter diflucan generic order diflucan
buy cytotec online: cytotec buy online usa – cytotec pills buy online
http://doxycycline.auction/# buy doxycycline 100mg
https://diflucan.pro/# buy diflucan canada
buy doxycycline without prescription uk doxycycline generic doxycycline hyc
buy cytotec online fast delivery: cytotec pills buy online – Misoprostol 200 mg buy online
http://cytotec24.com/# buy misoprostol over the counter
http://nolvadex.guru/# what happens when you stop taking tamoxifen
http://nolvadex.guru/# common side effects of tamoxifen
buy cytotec over the counter buy cytotec over the counter buy cytotec over the counter
http://cytotec24.com/# cytotec buy online usa
https://cipro.guru/# cipro 500mg best prices
how to buy doxycycline online 100mg doxycycline 100mg doxycycline
https://cipro.guru/# ciprofloxacin generic price
http://diflucan.pro/# diflucan 110 mg
http://doxycycline.auction/# odering doxycycline
diflucan generic cost diflucan 150 mg cost can you buy diflucan over the counter uk
http://diflucan.pro/# diflucan cream prescription
https://angelawhite.pro/# ?????? ????
https://lanarhoades.fun/# lana rhoades modeli
lana rhodes: lana rhoades modeli – lana rhodes
http://evaelfie.pro/# eva elfie izle
https://sweetiefox.online/# sweeti fox
Sweetie Fox video: Sweetie Fox – Sweetie Fox modeli
https://lanarhoades.fun/# lana rhoades
https://angelawhite.pro/# ?????? ????
http://sweetiefox.online/# Sweetie Fox filmleri
Sweetie Fox: Sweetie Fox video – sweety fox
https://sweetiefox.online/# Sweetie Fox
http://lanarhoades.fun/# lana rhoades izle
https://abelladanger.online/# abella danger filmleri
Angela White video: Angela White – ?????? ????
https://sweetiefox.online/# sweety fox
http://sweetiefox.online/# Sweetie Fox filmleri
http://angelawhite.pro/# Angela White video
Sweetie Fox filmleri: sweeti fox – sweety fox
https://abelladanger.online/# abella danger izle
http://evaelfie.pro/# eva elfie modeli
Angela Beyaz modeli: Angela White filmleri – Angela White video
http://sweetiefox.online/# sweety fox
http://evaelfie.pro/# eva elfie modeli
https://evaelfie.pro/# eva elfie
lana rhoades filmleri: lana rhoades modeli – lana rhoades izle
http://evaelfie.pro/# eva elfie
http://lanarhoades.fun/# lana rhoades filmleri
Angela White izle: Abella Danger – abella danger filmleri
http://evaelfie.pro/# eva elfie izle
https://evaelfie.pro/# eva elfie modeli
http://abelladanger.online/# abella danger izle
sweety fox: Sweetie Fox – Sweetie Fox video
https://sweetiefox.online/# swetie fox
http://sweetiefox.online/# Sweetie Fox izle
Angela White izle: abella danger izle – abella danger filmleri
http://abelladanger.online/# Abella Danger
http://angelawhite.pro/# Angela Beyaz modeli
http://lanarhoades.fun/# lana rhoades video
https://evaelfie.pro/# eva elfie
http://sweetiefox.online/# Sweetie Fox modeli
Angela White izle: Angela White video – Angela White video
http://evaelfie.pro/# eva elfie izle
https://evaelfie.pro/# eva elfie filmleri
sweety fox: Sweetie Fox izle – Sweetie Fox
http://sweetiefox.online/# swetie fox
?????? ????: ?????? ???? – Angela White izle
https://abelladanger.online/# abella danger filmleri
sweetie fox full video: sweetie fox cosplay – sweetie fox
https://sweetiefox.pro/# ph sweetie fox
eva elfie full videos: eva elfie hot – eva elfie full videos
https://miamalkova.life/# mia malkova hd
dating services melbourne: http://sweetiefox.pro/# sweetie fox full video
mia malkova videos: mia malkova movie – mia malkova new video
http://miamalkova.life/# mia malkova only fans
eva elfie new video: eva elfie – eva elfie hot
mia malkova latest: mia malkova new video – mia malkova
https://miamalkova.life/# mia malkova latest
ourtime login: https://lanarhoades.pro/# lana rhoades pics
eva elfie full video: eva elfie videos – eva elfie full video
sweetie fox video: sweetie fox full – sweetie fox cosplay
https://lanarhoades.pro/# lana rhoades unleashed
lana rhoades full video: lana rhoades boyfriend – lana rhoades full video
sex dating sights: https://miamalkova.life/# mia malkova movie
https://sweetiefox.pro/# sweetie fox cosplay
ph sweetie fox: sweetie fox full – fox sweetie
fox sweetie: fox sweetie – fox sweetie
lana rhoades videos: lana rhoades hot – lana rhoades boyfriend
Your article helped me a lot, is there any more related content? Thanks!
http://lanarhoades.pro/# lana rhoades videos
online dating and personals at: https://sweetiefox.pro/# ph sweetie fox
lana rhoades full video: lana rhoades boyfriend – lana rhoades solo
http://sweetiefox.pro/# sweetie fox full video
eva elfie full video: eva elfie videos – eva elfie hot
fox sweetie: fox sweetie – fox sweetie
https://lanarhoades.pro/# lana rhoades
christian dating: http://lanarhoades.pro/# lana rhoades pics
eva elfie photo: eva elfie – eva elfie hd
http://sweetiefox.pro/# sweetie fox cosplay
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
sweetie fox: ph sweetie fox – sweetie fox video
mia malkova new video: mia malkova photos – mia malkova latest
https://evaelfie.site/# eva elfie hd
lesbian mature: http://lanarhoades.pro/# lana rhoades videos
sweetie fox full video: sweetie fox cosplay – sweetie fox cosplay
https://lanarhoades.pro/# lana rhoades unleashed
eva elfie full video: eva elfie new video – eva elfie hd
http://aviatorjogar.online/# aviator jogo
http://jogodeaposta.fun/# jogo de aposta
aviator bet malawi: aviator game online – play aviator
http://aviatoroyunu.pro/# aviator oyna
aplicativo de aposta: jogo de aposta online – deposito minimo 1 real
pin up aviator: jogar aviator online – estrela bet aviator
depakote classification
http://aviatormocambique.site/# aviator
https://aviatormalawi.online/# aviator bet malawi login
aviator oyna: aviator oyunu – aviator oyunu
https://aviatoroyunu.pro/# aviator hilesi
site de apostas: jogo de aposta online – melhor jogo de aposta
http://aviatoroyunu.pro/# aviator
https://aviatoroyunu.pro/# aviator
melhor jogo de aposta para ganhar dinheiro: aviator jogo de aposta – depósito mínimo 1 real
jogar aviator Brasil: aviator jogar – jogar aviator Brasil
http://aviatormalawi.online/# aviator game online
https://pinupcassino.pro/# pin up bet
aviator ghana: play aviator – aviator
pin-up casino entrar: pin-up – pin up
jogar aviator Brasil: aviator betano – aviator bet
jogo de aposta: melhor jogo de aposta – jogo de aposta online
aviator betting game: aviator game – play aviator
https://aviatorghana.pro/# play aviator
aviator game online: play aviator – aviator bet malawi login
aviator bahis: aviator oyna slot – aviator hilesi
aviator game online: aviator betting game – aviator bet
jogar aviator: como jogar aviator – aviator mz
pin-up: pin up casino – pin-up casino
http://aviatormalawi.online/# aviator bet
buy cheap zithromax online – https://azithromycin.pro/zithromax-500-mg-coupon.html zithromax 1000 mg pills
aviator game: aviator game bet – aviator bet
where can i get zithromax over the counter: zithromax dosing for pediatrics zithromax online paypal
https://aviatormocambique.site/# aviator
aviator game: aviator betting game – aviator sportybet ghana
como jogar aviator: aviator – jogar aviator
zithromax 500 mg: can you drink with zithromax zithromax capsules 250mg
jogar aviator online: pin up aviator – aviator game
buy prescription drugs from canada cheap: Cheapest drug prices Canada – canadian drugstore online canadianpharm.store
http://canadianpharmlk.shop/# canadian online drugs canadianpharm.store
india pharmacy mail order indian pharmacy cheapest online pharmacy india indianpharm.store
indian pharmacy paypal: world pharmacy india – top 10 online pharmacy in india indianpharm.store
medicine in mexico pharmacies: Mexico pharmacy price list – pharmacies in mexico that ship to usa mexicanpharm.shop
https://canadianpharmlk.com/# is canadian pharmacy legit canadianpharm.store
mexican mail order pharmacies mexican pharmacy medicine in mexico pharmacies mexicanpharm.shop
https://canadianpharmlk.shop/# canada cloud pharmacy canadianpharm.store
reputable mexican pharmacies online: Mexico pharmacy online – mexico drug stores pharmacies mexicanpharm.shop
https://indianpharm24.com/# п»їlegitimate online pharmacies india indianpharm.store
world pharmacy india: Online medicine home delivery – india online pharmacy indianpharm.store
http://mexicanpharm24.com/# mexican rx online mexicanpharm.shop
http://mexicanpharm24.shop/# buying from online mexican pharmacy mexicanpharm.shop
top 10 pharmacies in india: Online India pharmacy – indianpharmacy com indianpharm.store
http://mexicanpharm24.shop/# mexico drug stores pharmacies mexicanpharm.shop
http://indianpharm24.com/# indianpharmacy com indianpharm.store
http://canadianpharmlk.com/# best mail order pharmacy canada canadianpharm.store
purple pharmacy mexico price list Medicines Mexico п»їbest mexican online pharmacies mexicanpharm.shop
https://mexicanpharm24.com/# medication from mexico pharmacy mexicanpharm.shop
best online pharmacies in mexico: order online from a Mexican pharmacy – mexico drug stores pharmacies mexicanpharm.shop
http://canadianpharmlk.com/# canadian pharmacy review canadianpharm.store
mexican drugstore online: order online from a Mexican pharmacy – mexican drugstore online mexicanpharm.shop
https://indianpharm24.com/# top 10 online pharmacy in india indianpharm.store
medicine in mexico pharmacies: Mexico pharmacy online – reputable mexican pharmacies online mexicanpharm.shop
http://mexicanpharm24.com/# п»їbest mexican online pharmacies mexicanpharm.shop
https://indianpharm24.com/# indian pharmacies safe indianpharm.store
https://canadianpharmlk.com/# escrow pharmacy canada canadianpharm.store
best canadian online pharmacy: CIPA approved pharmacies – canadian pharmacy phone number canadianpharm.store
https://mexicanpharm24.shop/# mexico pharmacy mexicanpharm.shop
best online pharmacies in mexico mexican pharmacy reputable mexican pharmacies online mexicanpharm.shop
https://mexicanpharm24.com/# buying prescription drugs in mexico online mexicanpharm.shop
https://canadianpharmlk.shop/# legit canadian pharmacy online canadianpharm.store
https://indianpharm24.com/# india online pharmacy indianpharm.store
mexico pharmacies prescription drugs: best online pharmacies in mexico – mexican pharmaceuticals online mexicanpharm.shop
canadapharmacyonline com: Canadian pharmacy prices – vipps canadian pharmacy canadianpharm.store
http://canadianpharmlk.shop/# legit canadian online pharmacy canadianpharm.store
https://canadianpharmlk.shop/# trusted canadian pharmacy canadianpharm.store
get cheap clomid: clomid 100mg – can you get cheap clomid online
where to get clomid no prescription: cheap clomid for sale – where buy cheap clomid without rx
cost of generic clomid: clomid dosage for men – how can i get clomid no prescription
https://amoxilst.pro/# purchase amoxicillin online without prescription
5 mg prednisone daily: prednisone killed my dog – buying prednisone without prescription
generic prednisone for sale: prednisone price australia – prednisone 10 mg
amoxicillin 500mg capsule buy online where can you buy amoxicillin over the counter order amoxicillin online uk
http://clomidst.pro/# where buy clomid prices
buy prednisone without a prescription best price: prednisone tabs 20 mg – prednisone best price
how to buy generic clomid: how to get generic clomid – generic clomid no prescription
amoxicillin no prescription: amoxicillin 500 mg capsule – buy cheap amoxicillin
prednisone over the counter australia: prednisone 20 tablet – prednisone 0.5 mg
http://amoxilst.pro/# price of amoxicillin without insurance
amoxicillin 500mg over the counter: how much is amoxicillin without insurance – buy amoxicillin 500mg capsules uk
can i buy clomid without insurance: clomid while on trt – can i get cheap clomid pills
prednisone 20mg tablets where to buy: prednisone for sinus infection – can you buy prednisone
can i get cheap clomid no prescription: cost clomid tablets – buying generic clomid prices
https://clomidst.pro/# can you get clomid without insurance
generic amoxicillin 500mg amoxicillin 875 mg tablet buy amoxil
cost of clomid without dr prescription: how to get clomid for sale – can you get generic clomid without dr prescription
can i buy prednisone over the counter in usa: prednisone in uk – prednisone 25mg from canada
amoxicillin 500: ampicillin vs amoxicillin – how much is amoxicillin
http://amoxilst.pro/# amoxicillin capsule 500mg price
where to get clomid price: pct clomid – can i get generic clomid without dr prescription
amoxicillin 500mg no prescription: amoxicillin-clavulanate – amoxacillian without a percription
60 mg prednisone daily: alcohol and prednisone – prednisone 20 mg tablet
generic clomid: cost of clomid no prescription – where to buy generic clomid pills
https://amoxilst.pro/# amoxicillin for sale
can i order cheap clomid: where to buy generic clomid pill – cheap clomid without prescription
online order prednisone 10mg online order prednisone 10mg prednisone 50 mg tablet canada
online ed drugs boner pills online order ed pills
http://onlinepharmacy.cheap/# cheapest pharmacy to fill prescriptions with insurance
buy erectile dysfunction medication: buy ed meds – cheap boner pills
best no prescription online pharmacies: canadian pharmacy online no prescription – canadian prescription drugstore reviews
https://onlinepharmacy.cheap/# drugstore com online pharmacy prescription drugs
erectile dysfunction online prescription: ed treatments online – erectile dysfunction online
canadian prescription: canada mail order prescriptions – pharmacy with no prescription
online pharmacy prescription: online pharmacy delivery – no prescription pharmacy paypal
https://pharmnoprescription.pro/# buy prescription drugs without a prescription
http://edpills.guru/# low cost ed meds
pharmacy no prescription required: no prescription drugs online – best online pharmacy that does not require a prescription in india
ed online prescription cheap ed drugs cheapest online ed treatment
buying prescription medications online: best online pharmacies without prescription – quality prescription drugs canada
https://edpills.guru/# online prescription for ed
buy pills without prescription: buy medication online no prescription – best online prescription
online pharmacy no prescription: Online pharmacy USA – online canadian pharmacy coupon
buy drugs online without prescription: buy prescription drugs on line – non prescription online pharmacy india
http://edpills.guru/# erectile dysfunction online
how to get ed meds online: erectile dysfunction medicine online – cheapest online ed meds
ed treatment online: edmeds – erectile dysfunction medicine online
us pharmacy no prescription: online pharmacy delivery – canadian pharmacy world coupon code
https://pharmnoprescription.pro/# medicine with no prescription
uk pharmacy no prescription Cheapest online pharmacy offshore pharmacy no prescription
canadian pharmacy without prescription: online pharmacy delivery – canadian pharmacy no prescription
https://pharmnoprescription.pro/# legitimate online pharmacy no prescription
online erectile dysfunction prescription: ed medicines – cheapest ed meds
cheap ed: ed meds cheap – erectile dysfunction pills for sale
http://canadianpharm.guru/# cross border pharmacy canada
canadian pharmacy meds: pharmacy canadian – canadapharmacyonline com
mexican drugstore online: mexico pharmacies prescription drugs – buying from online mexican pharmacy
https://mexicanpharm.online/# mexico drug stores pharmacies
canadian pharmacy 24h com safe canadian pharmacy online canadian online drugs
buy medication online without prescription: online drugstore no prescription – buy medication online with prescription
http://pharmacynoprescription.pro/# online medication no prescription
http://indianpharm.shop/# buy prescription drugs from india
medicine in mexico pharmacies: reputable mexican pharmacies online – mexico pharmacies prescription drugs
cheapest online pharmacy india: buy prescription drugs from india – world pharmacy india
top online pharmacy india: indian pharmacy – buy medicines online in india
http://mexicanpharm.online/# mexican mail order pharmacies
mexican mail order pharmacies: purple pharmacy mexico price list – medication from mexico pharmacy
my canadian pharmacy review: best canadian pharmacy – safe canadian pharmacy
п»їbest mexican online pharmacies: mexican rx online – п»їbest mexican online pharmacies
https://mexicanpharm.online/# purple pharmacy mexico price list
http://indianpharm.shop/# mail order pharmacy india
canadian drugs pharmacy: real canadian pharmacy – my canadian pharmacy
india online pharmacy: online shopping pharmacy india – indian pharmacy online
mexican rx online: mexican rx online – medication from mexico pharmacy
http://mexicanpharm.online/# mexico pharmacy
precription drugs from canada: canadian pharmacy checker – canadadrugpharmacy com
prescription from canada: canadian and international prescription service – buying drugs without prescription
https://mexicanpharm.online/# mexican online pharmacies prescription drugs
canadian pharmacy 365: canadian pharmacy service – canadian drug pharmacy
canadian pharmacy prices: maple leaf pharmacy in canada – canadian neighbor pharmacy
mexican mail order pharmacies mexican online pharmacies prescription drugs mexican rx online
https://pharmacynoprescription.pro/# best website to buy prescription drugs
canada drug pharmacy: safe reliable canadian pharmacy – canada rx pharmacy
Online medicine home delivery: Online medicine home delivery – online pharmacy india
https://indianpharm.shop/# indian pharmacy online
mexican pharmacy: buying prescription drugs in mexico – mexican border pharmacies shipping to usa
medication from mexico pharmacy: buying prescription drugs in mexico – medicine in mexico pharmacies
mexican drugstore online: mexican online pharmacies prescription drugs – medicine in mexico pharmacies
canadian pharmacy: canadapharmacyonline – canadian pharmacy in canada
trusted canadian pharmacy: buying from canadian pharmacies – canadian pharmacy 1 internet online drugstore
medication online without prescription: canadian pharmacy without prescription – canadian rx prescription drugstore
best online pharmacies in mexico medication from mexico pharmacy medicine in mexico pharmacies
http://indianpharm.shop/# buy medicines online in india
https://pharmacynoprescription.pro/# overseas online pharmacy-no prescription
reputable indian online pharmacy: legitimate online pharmacies india – online shopping pharmacy india
buying drugs from canada: canadian pharmacy victoza – northwest canadian pharmacy
canadapharmacyonline legit: canadian pharmacy meds – best canadian pharmacy online
http://pharmacynoprescription.pro/# canada mail order prescriptions
cheap drugs no prescription: buying prescription drugs from canada online – best online pharmacy without prescriptions
mexican border pharmacies shipping to usa: buying from online mexican pharmacy – mexican border pharmacies shipping to usa
http://canadianpharm.guru/# canadian pharmacies
canadian pharmacy cheap canadian drug canadian medications
https://canadianpharm.guru/# thecanadianpharmacy
canadian online pharmacy reviews: canadian drug stores – reputable canadian pharmacy
reputable mexican pharmacies online: mexican online pharmacies prescription drugs – best mexican online pharmacies
canadian prescription: online pharmacy no prescriptions – online prescription canada
http://mexicanpharm.online/# reputable mexican pharmacies online
mexican rx online: medicine in mexico pharmacies – buying from online mexican pharmacy
https://mexicanpharm.online/# buying prescription drugs in mexico
buy prescription online: buying prescription drugs from canada online – no prescription medicines
best online pharmacy without prescription: mail order prescriptions from canada – canadian pharmacy without prescription
mexican pharmacy: mexican pharmaceuticals online – reputable mexican pharmacies online
Online medicine order: india pharmacy mail order – indian pharmacy online
http://indianpharm.shop/# pharmacy website india
cheap drugs no prescription pharmacy no prescription required medicine with no prescription
buying prescription drugs in india: canadian pharmacy no prescription needed – online pharmacies no prescription
http://canadianpharm.guru/# canadian pharmacy
buy drugs online without prescription: online pharmacy not requiring prescription – no prescription pharmacy
Online medicine order: reputable indian pharmacies – reputable indian pharmacies
http://pharmacynoprescription.pro/# online canadian pharmacy no prescription
canadian pharmacy online ship to usa: safe canadian pharmacy – canadian drug prices
sweet bonanza bahis: sweet bonanza free spin demo – sweet bonanza taktik
http://slotsiteleri.guru/# canli slot siteleri
https://gatesofolympus.auction/# gates of olympus s?rlar?
gates of olympus 1000 demo: gates of olympus taktik – pragmatic play gates of olympus
http://aviatoroyna.bid/# aviator oyna
aviator oyunu giris: aviator hilesi – aviator oyunu 100 tl
http://gatesofolympus.auction/# gates of olympus oyna ücretsiz
pin up 7/24 giris: pin up – pin up casino guncel giris
http://gatesofolympus.auction/# gates of olympus slot
http://slotsiteleri.guru/# en cok kazandiran slot siteleri
https://aviatoroyna.bid/# aviator hilesi ücretsiz
gates of olympus taktik: gates of olympus 1000 demo – gates of olympus 1000 demo
en guvenilir slot siteleri: slot siteleri bonus veren – en yeni slot siteleri
https://pinupgiris.fun/# pin up giris
gates of olympus demo turkce oyna: gates of olympus oyna ucretsiz – gates of olympus
https://aviatoroyna.bid/# aviator nasil oynanir
https://aviatoroyna.bid/# aviator sinyal hilesi ücretsiz
https://gatesofolympus.auction/# gates of olympus oyna ucretsiz
pin-up casino giris: pin-up casino indir – pin up 7/24 giris
https://pinupgiris.fun/# pin-up giris
sweet bonanza nas?l oynan?r: sweet bonanza 90 tl – sweet bonanza guncel
aviator oyunu 10 tl: aviator giris – aviator
https://slotsiteleri.guru/# slot siteleri
http://slotsiteleri.guru/# slot oyunlari siteleri
aviator oyna 100 tl: aviator oyunu 100 tl – aviator
http://slotsiteleri.guru/# deneme bonusu veren slot siteleri
https://aviatoroyna.bid/# aviator oyna
gates of olympus 1000 demo: gates of olympus s?rlar? – gates of olympus giris
https://sweetbonanza.bid/# sweet bonanza kazanç
pin up casino giris: pin up casino indir – aviator pin up
https://gatesofolympus.auction/# pragmatic play gates of olympus
sweet bonanza slot: sweet bonanza slot demo – sweet bonanza bahis
https://sweetbonanza.bid/# sweet bonanza slot
bonus veren slot siteleri: slot oyunlar? siteleri – canl? slot siteleri
ashwagandha for stress
indianpharmacy com: india pharmacy – reputable indian online pharmacy
top 10 pharmacies in india Healthcare and medicines from India top 10 online pharmacy in india
п»їlegitimate online pharmacies india: Healthcare and medicines from India – indian pharmacies safe
https://canadianpharmacy24.store/# reputable canadian pharmacy
mexico pharmacies prescription drugs mexican pharmacy mexico pharmacy
buying from canadian pharmacies: Certified Canadian Pharmacy – best canadian online pharmacy
purple pharmacy mexico price list: mexico pharmacy – mexico pharmacies prescription drugs
mexican online pharmacies prescription drugs mexican pharmacy mexican drugstore online
india online pharmacy: Generic Medicine India to USA – buy prescription drugs from india
buy medicines online in india Generic Medicine India to USA top 10 online pharmacy in india
canadian pharmacy 24: Large Selection of Medications – legitimate canadian pharmacy
Online medicine home delivery: reputable indian online pharmacy – india pharmacy mail order
canadian pharmacy 24h com safe: Large Selection of Medications – canadian 24 hour pharmacy
safe canadian pharmacies: canadian king pharmacy – canadian pharmacy meds reviews
reputable indian online pharmacy Generic Medicine India to USA indian pharmacy online
reputable indian online pharmacy: indian pharmacy – Online medicine order
buy prescription drugs from india: indian pharmacy – india pharmacy mail order
mexico pharmacies prescription drugs: Mexican Pharmacy Online – mexican mail order pharmacies
top 10 online pharmacy in india Healthcare and medicines from India Online medicine home delivery
legal to buy prescription drugs from canada: canadian pharmacy 24 – canadian pharmacy meds
mexico pharmacies prescription drugs: mexican border pharmacies shipping to usa – buying prescription drugs in mexico online
https://indianpharmacy.icu/# indian pharmacy paypal
indian pharmacy online: reputable indian pharmacies – buy medicines online in india
medicine in mexico pharmacies mexico pharmacy mexican online pharmacies prescription drugs
mexico drug stores pharmacies: medicine in mexico pharmacies – best online pharmacies in mexico
top online pharmacy india: Generic Medicine India to USA – world pharmacy india
pharmacy canadian superstore: Prescription Drugs from Canada – pet meds without vet prescription canada
buying prescription drugs in mexico online: Mexican Pharmacy Online – mexico drug stores pharmacies
buy prescription drugs from india Healthcare and medicines from India cheapest online pharmacy india
indian pharmacy paypal indian pharmacy delivery india online pharmacy
indian pharmacy online: Healthcare and medicines from India – buy prescription drugs from india
https://canadianpharmacy24.store/# canadian pharmacy reviews
pharmacy com canada Licensed Canadian Pharmacy canadian pharmacy 24 com
mexican border pharmacies shipping to usa: Online Pharmacies in Mexico – mexico drug stores pharmacies
Online medicine order: Healthcare and medicines from India – pharmacy website india
canadian pharmacy sarasota: Certified Canadian Pharmacy – real canadian pharmacy
canadian pharmacy ed medications: canadian pharmacy near me – canadianpharmacyworld
how can i get cheap clomid without dr prescription where can i get cheap clomid without prescription how to buy cheap clomid now
http://clomidall.com/# where can i buy generic clomid without dr prescription
http://amoxilall.shop/# order amoxicillin no prescription
cost of prednisone 10mg tablets 5 mg prednisone daily prednisone price
prednisone medication: buy prednisone online from canada – how much is prednisone 10mg
https://zithromaxall.com/# zithromax price canada
buying generic clomid price: get cheap clomid without a prescription – buying cheap clomid without dr prescription
buying clomid tablets where to buy generic clomid without prescription buy clomid no prescription
https://clomidall.com/# how can i get generic clomid
http://zithromaxall.com/# zithromax 250
http://clomidall.com/# can i get generic clomid no prescription
how to buy amoxicillin online amoxicillin no prescipion amoxicillin 500 mg price
https://prednisoneall.shop/# buy prednisone with paypal canada
http://clomidall.shop/# can i get generic clomid no prescription
zithromax prescription in canada zithromax 250 mg tablet price zithromax azithromycin
buy prednisone 1 mg mexico: prednisone 10 mg tablet cost – prednisone 5 mg tablet price
https://clomidall.shop/# where to buy cheap clomid
can i order cheap clomid tablets: where can i buy clomid without rx – where to get generic clomid without rx
http://prednisoneall.shop/# generic prednisone for sale
where can i purchase zithromax online zithromax over the counter canada zithromax cost australia
https://prednisoneall.shop/# prednisone sale
https://amoxilall.com/# buy amoxicillin online no prescription
buy zithromax canada zithromax drug generic zithromax over the counter
https://clomidall.com/# where can i buy clomid without rx
https://amoxilall.com/# amoxicillin 500mg capsules price
http://zithromaxall.shop/# buy zithromax online cheap
amoxicillin buy canada where can you get amoxicillin where can i buy amoxicillin over the counter uk
prednisone pak: cheap prednisone online – buy prednisone online uk
http://amoxilall.com/# 875 mg amoxicillin cost
prednisone price australia prednisone tablets canada prednisone in canada
prednisone 50 mg tablet canada: prednisone 12 mg – 100 mg prednisone daily
https://clomidall.com/# can i order generic clomid no prescription
http://clomidall.com/# how to buy cheap clomid online
http://prednisoneall.com/# order prednisone
buy amoxicillin 500mg online 875 mg amoxicillin cost amoxicillin 250 mg
https://sildenafiliq.com/# cheapest viagra
п»їkamagra Kamagra gel super kamagra
buy cialis pill: cheapest cialis – Cialis 20mg price
https://tadalafiliq.com/# buy cialis pill
Buy Tadalafil 5mg: Generic Cialis without a doctor prescription – cialis for sale
over the counter sildenafil: best price on viagra – buy Viagra over the counter
buy kamagra online usa Sildenafil Oral Jelly Kamagra 100mg
Kamagra 100mg price: Kamagra 100mg – super kamagra
http://kamagraiq.shop/# Kamagra tablets
http://sildenafiliq.xyz/# Sildenafil 100mg price
super kamagra Sildenafil Oral Jelly cheap kamagra
Sildenafil 100mg price: Cheap Viagra 100mg – Cheap Viagra 100mg
Generic Cialis without a doctor prescription: Buy Cialis online – Generic Tadalafil 20mg price
Kamagra 100mg price: Kamagra Oral Jelly Price – Kamagra 100mg price
http://tadalafiliq.com/# Generic Cialis price
Generic Cialis without a doctor prescription cialis best price Tadalafil price
buy Viagra over the counter: generic ed pills – Sildenafil Citrate Tablets 100mg
Generic Cialis price: Generic Tadalafil 20mg price – Tadalafil price
Generic Cialis without a doctor prescription Buy Tadalafil 10mg Cheap Cialis
https://tadalafiliq.shop/# Buy Tadalafil 5mg
kamagra: Kamagra Iq – sildenafil oral jelly 100mg kamagra
http://tadalafiliq.com/# buy cialis pill
http://kamagraiq.com/# Kamagra 100mg price
Buy Tadalafil 10mg cialis best price Buy Cialis online
Kamagra Oral Jelly: kamagra best price – buy kamagra online usa
https://tadalafiliq.shop/# Cialis 20mg price
cheapest viagra: generic ed pills – viagra canada
buy cialis pill: cheapest cialis – Generic Cialis price
Buy Cialis online tadalafil iq buy cialis pill
cheapest cialis: tadalafil iq – cialis generic
https://sildenafiliq.xyz/# п»їBuy generic 100mg Viagra online
https://sildenafiliq.xyz/# Cheap generic Viagra online
https://sildenafiliq.xyz/# Cheap generic Viagra online
Generic Cialis without a doctor prescription: Buy Cialis online – Buy Cialis online
Cialis without a doctor prescription tadalafil iq Generic Cialis without a doctor prescription
https://sildenafiliq.xyz/# generic sildenafil
Buy generic 100mg Viagra online: best price on viagra – cheap viagra
https://tadalafiliq.com/# Generic Cialis price
п»їkamagra Sildenafil Oral Jelly Kamagra Oral Jelly
Kamagra 100mg: Kamagra Oral Jelly Price – cheap kamagra
https://kamagraiq.com/# Kamagra 100mg
Viagra online price: cheapest viagra – generic sildenafil
Buy Tadalafil 5mg: Generic Tadalafil 20mg price – Buy Tadalafil 10mg
Cialis 20mg price Generic Tadalafil 20mg price Tadalafil Tablet
http://tadalafiliq.com/# Cialis over the counter
Kamagra tablets: Kamagra Oral Jelly Price – Kamagra 100mg price
https://tadalafiliq.com/# Tadalafil price
http://kamagraiq.com/# cheap kamagra
canadian pharmacy canadian pharmacy canadian pharmacy ltd
https://mexicanpharmgrx.com/# mexico drug stores pharmacies
canadapharmacyonline com: Canadian pharmacy prices – canadian online pharmacy reviews
best canadian online pharmacy: canadian pharmacy – online canadian pharmacy reviews
buying prescription drugs in mexico medication from mexico pharmacy mexican pharmacy
http://indianpharmgrx.shop/# indian pharmacies safe
https://indianpharmgrx.shop/# reputable indian online pharmacy
buy prescription drugs from india: Generic Medicine India to USA – indianpharmacy com
top online pharmacy india Healthcare and medicines from India online pharmacy india
http://mexicanpharmgrx.shop/# reputable mexican pharmacies online
buying from online mexican pharmacy: Mexico drugstore – mexican drugstore online
best online pharmacy india india pharmacy mail order india online pharmacy
https://canadianpharmgrx.xyz/# canadapharmacyonline legit
canadian family pharmacy: precription drugs from canada – canadian pharmacy 24h com
https://mexicanpharmgrx.com/# mexico pharmacy
reliable canadian online pharmacy Canadian pharmacy prices canadian online drugstore
buy medicines online in india: indian pharmacy delivery – top online pharmacy india
http://indianpharmgrx.com/# indian pharmacy
top 10 pharmacies in india: Healthcare and medicines from India – п»їlegitimate online pharmacies india
top online pharmacy india Generic Medicine India to USA buy prescription drugs from india
https://mexicanpharmgrx.com/# mexican drugstore online
india pharmacy mail order Healthcare and medicines from India п»їlegitimate online pharmacies india
https://mexicanpharmgrx.shop/# mexico drug stores pharmacies
mexican rx online: Mexico drugstore – pharmacies in mexico that ship to usa
india online pharmacy: indian pharmacy delivery – online pharmacy india
https://mexicanpharmgrx.shop/# mexico pharmacies prescription drugs
п»їlegitimate online pharmacies india Healthcare and medicines from India online pharmacy india
https://canadianpharmgrx.com/# canada drug pharmacy
mexican rx online: Pills from Mexican Pharmacy – п»їbest mexican online pharmacies
www canadianonlinepharmacy: Canadian pharmacy prices – canadian discount pharmacy
buying from online mexican pharmacy mexican pharmacy mexico pharmacies prescription drugs
http://canadianpharmgrx.xyz/# canadian mail order pharmacy
http://mexicanpharmgrx.com/# mexican online pharmacies prescription drugs
77 canadian pharmacy canadian pharmacy reliable canadian pharmacy reviews
canada rx pharmacy: Canada pharmacy online – adderall canadian pharmacy
http://indianpharmgrx.shop/# online pharmacy india
cytotec buy online usa: buy cytotec in usa – buy cytotec online fast delivery
buy cytotec pills online cheap: buy cytotec online fast delivery – order cytotec online
tamoxifen postmenopausal alternative to tamoxifen nolvadex d
diflucan drug coupon: diflucan price south africa – can i purchase diflucan over the counter
cytotec online: Misoprostol 200 mg buy online – Cytotec 200mcg price
http://misoprostol.top/# buy cytotec pills online cheap
diflucan 150 mg tablet diflucan otc uk diflucan pills for sale
tamoxifen hip pain: nolvadex vs clomid – nolvadex estrogen blocker
diflucan rx online: how much is diflucan – diflucan online prescription
who should take tamoxifen tamoxifen cancer tamoxifen effectiveness
diflucan online no prescription: over the counter diflucan 150 – where can i buy diflucan over the counter
is nolvadex legal tamoxifen warning how to prevent hair loss while on tamoxifen
cytotec abortion pill: buy cytotec – buy cytotec online
is nolvadex legal: tamoxifen rash – does tamoxifen cause menopause
Abortion pills online: buy cytotec in usa – Misoprostol 200 mg buy online
buy cytotec over the counter purchase cytotec buy cytotec online
diflucan 150 mg daily: buy diflucan online no prescription – diflucan otc uk
where can i buy diflucan over the counter: where to get diflucan without a prescription – cost of diflucan over the counter
diflucan medication prescription: diflucan canada coupon – how much is diflucan
antibiotics cipro buy cipro online canada п»їcipro generic
buy doxycycline cheap: generic doxycycline – doxycycline tetracycline
buy cytotec buy cytotec online Misoprostol 200 mg buy online
buy cipro: ciprofloxacin order online – cipro 500mg best prices
http://diflucan.icu/# buy diflucan yeast infection
diflucan without get a prescription online: diflucan 100 – buying diflucan without prescription
buy cytotec over the counter: cytotec online – buy cytotec in usa
buy doxycycline online 270 tabs: doxycycline hyc 100mg – buy doxycycline monohydrate
diflucan pill uk how to buy diflucan generic for diflucan
ciprofloxacin mail online: ciprofloxacin over the counter – cipro 500mg best prices
tamoxifen citrate pct where to get nolvadex arimidex vs tamoxifen bodybuilding
generic for doxycycline: doxycycline medication – buy doxycycline 100mg
buy cipro: ciprofloxacin over the counter – where can i buy cipro online
nolvadex gynecomastia tamoxifen estrogen tamoxifen depression
https://stromectola.top/# minocycline 100mg pills online
can i buy cheap clomid online order cheap clomid now can i buy clomid for sale
50 mg prednisone tablet: prednisone over the counter cost – prednisone 100 mg
buy zithromax without prescription online: zithromax 600 mg tablets – can you buy zithromax online
buy amoxicillin over the counter uk: buy amoxil – amoxicillin order online no prescription
amoxicillin 500 coupon amoxicillin capsule 500mg price amoxicillin pills 500 mg
average cost of prednisone: prednisone acetate – prednisone 40 mg daily
buy amoxicillin online mexico: over the counter amoxicillin canada – buy amoxicillin without prescription
https://prednisonea.store/# buy prednisone online no prescription
http://stromectola.top/# ivermectin over the counter
purchase amoxicillin 500 mg order amoxicillin 500mg amoxicillin 200 mg tablet
zithromax antibiotic: where can you buy zithromax – zithromax online pharmacy canada
can i buy amoxicillin online: amoxicillin medicine over the counter – amoxicillin 500 mg for sale
http://stromectola.top/# ivermectin 1mg
ivermectin lice oral buy oral ivermectin ivermectin 6mg dosage
buy zithromax without presc: zithromax cost uk – zithromax 250 price
http://amoxicillina.top/# buy amoxicillin 500mg
amoxicillin buy online canada: buy amoxicillin online mexico – where to buy amoxicillin 500mg without prescription
can you buy prednisone over the counter: buy prednisone 40 mg – where can i buy prednisone without a prescription
how to buy amoxicillin online buy amoxicillin online uk can i purchase amoxicillin online
zithromax 1000 mg pills: zithromax antibiotic without prescription – zithromax over the counter canada
http://prednisonea.store/# fast shipping prednisone
https://medicationnoprescription.pro/# prescription drugs online canada
http://onlinepharmacyworld.shop/# rx pharmacy coupons
overseas pharmacy no prescription prescription drugs from canada cheapest pharmacy for prescriptions
ed medications online: what is the cheapest ed medication – ed meds cheap
https://onlinepharmacyworld.shop/# canadian online pharmacy no prescription
https://edpill.top/# order ed pills
drugstore com online pharmacy prescription drugs cheapest pharmacy to get prescriptions filled prescription drugs from canada
ed meds online: how to get ed pills – order ed pills
http://edpill.top/# discount ed meds
best online ed pills: buying ed pills online – cheapest online ed meds
https://medicationnoprescription.pro/# canadian prescriptions in usa
https://onlinepharmacyworld.shop/# overseas pharmacy no prescription
https://edpill.top/# where to buy erectile dysfunction pills
canadian pharmacy without a prescription: buy drugs without prescription – mexican prescription drugs online
online drugs no prescription canadian and international prescription service canadian rx prescription drugstore
https://medicationnoprescription.pro/# no prescription medication
pills for ed online: ed pills for sale – cheap ed pills online
http://medicationnoprescription.pro/# best online prescription
buy medications without a prescription online pharmacy without a prescription canadian prescription prices
https://medicationnoprescription.pro/# online pharmacy not requiring prescription
https://medicationnoprescription.pro/# order medication without prescription
get ed meds today: where can i buy ed pills – online ed pharmacy
non prescription medicine pharmacy: canadian pharmacy without prescription – pharmacy coupons
non prescription online pharmacy india online meds without prescription buy medications without a prescription
http://onlinepharmacyworld.shop/# drugstore com online pharmacy prescription drugs
http://casinvietnam.shop/# game c? b?c online uy tin
http://casinvietnam.com/# casino tr?c tuy?n vi?t nam
http://casinvietnam.shop/# game c? b?c online uy tin
http://casinvietnam.com/# casino online uy tin
danh bai tr?c tuy?n casino tr?c tuy?n casino tr?c tuy?n uy tin
casino tr?c tuy?n uy tín: game c? b?c online uy tín – casino online uy tín
http://casinvietnam.com/# casino tr?c tuy?n uy tin
casino online uy tin casino online uy tin casino tr?c tuy?n vi?t nam
choi casino tr?c tuy?n trên di?n tho?i: web c? b?c online uy tín – casino tr?c tuy?n vi?t nam
http://casinvietnam.shop/# casino tr?c tuy?n
http://casinvietnam.com/# casino tr?c tuy?n vi?t nam
casino tr?c tuy?n: dánh bài tr?c tuy?n – casino tr?c tuy?n
casino tr?c tuy?n uy tín: casino tr?c tuy?n vi?t nam – web c? b?c online uy tín
http://casinvietnam.shop/# casino tr?c tuy?n uy tin
game c? b?c online uy tín: web c? b?c online uy tín – dánh bài tr?c tuy?n
http://casinvietnam.shop/# choi casino tr?c tuy?n tren di?n tho?i
dánh bài tr?c tuy?n: web c? b?c online uy tín – casino tr?c tuy?n
casino tr?c tuy?n uy tín: web c? b?c online uy tín – casino tr?c tuy?n vi?t nam
http://casinvietnam.shop/# choi casino tr?c tuy?n tren di?n tho?i
choi casino tr?c tuy?n trên di?n tho?i: casino tr?c tuy?n – casino online uy tín
http://casinvietnam.com/# casino tr?c tuy?n
web c? b?c online uy tín: dánh bài tr?c tuy?n – casino tr?c tuy?n vi?t nam
choi casino tr?c tuy?n trên di?n tho?i: choi casino tr?c tuy?n trên di?n tho?i – casino online uy tín
casino online uy tín: casino tr?c tuy?n vi?t nam – casino tr?c tuy?n uy tín
casino tr?c tuy?n uy tín: casino online uy tín – game c? b?c online uy tín
https://casinvietnam.shop/# casino tr?c tuy?n vi?t nam
casino tr?c tuy?n: casino tr?c tuy?n – game c? b?c online uy tín
https://mexicoph24.life/# mexican rx online
https://canadaph24.pro/# canadian pharmacy com
http://indiaph24.store/# Online medicine home delivery
http://indiaph24.store/# Online medicine home delivery
https://canadaph24.pro/# canadian pharmacy com
https://indiaph24.store/# cheapest online pharmacy india
https://mexicoph24.life/# medicine in mexico pharmacies
http://mexicoph24.life/# pharmacies in mexico that ship to usa
https://canadaph24.pro/# canadian pharmacy uk delivery
http://canadaph24.pro/# canadian pharmacy antibiotics
https://indiaph24.store/# indian pharmacy
https://canadaph24.pro/# reliable canadian online pharmacy
https://canadaph24.pro/# canada drugs reviews
https://canadaph24.pro/# canada online pharmacy
http://mexicoph24.life/# best mexican online pharmacies
https://canadaph24.pro/# canadadrugpharmacy com
https://mexicoph24.life/# purple pharmacy mexico price list
http://mexicoph24.life/# buying from online mexican pharmacy
http://canadaph24.pro/# canadian drugs pharmacy
https://mexicoph24.life/# mexican drugstore online
http://indiaph24.store/# indian pharmacies safe
http://canadaph24.pro/# canadian pharmacy reviews
https://indiaph24.store/# reputable indian online pharmacy
https://indiaph24.store/# cheapest online pharmacy india
https://mexicoph24.life/# mexican pharmacy
https://mexicoph24.life/# medicine in mexico pharmacies
https://mexicoph24.life/# mexican border pharmacies shipping to usa
http://canadaph24.pro/# pharmacy canadian
http://canadaph24.pro/# safe canadian pharmacy
https://indiaph24.store/# reputable indian online pharmacy
http://indiaph24.store/# cheapest online pharmacy india
https://canadaph24.pro/# canadian pharmacy
http://mexicoph24.life/# best online pharmacies in mexico
http://canadaph24.pro/# canadian drug stores
https://canadaph24.pro/# recommended canadian pharmacies
https://mexicoph24.life/# best mexican online pharmacies
http://mexicoph24.life/# mexican border pharmacies shipping to usa
http://canadaph24.pro/# best canadian pharmacy
https://mexicoph24.life/# buying from online mexican pharmacy
https://mexicoph24.life/# mexican drugstore online
https://canadaph24.pro/# canadian pharmacies online
http://mexicoph24.life/# purple pharmacy mexico price list
http://canadaph24.pro/# best online canadian pharmacy
top online pharmacy india https://indiaph24.store/# india pharmacy
п»їlegitimate online pharmacies india
indian pharmacy indian pharmacy best india pharmacy
http://canadaph24.pro/# canadian pharmacy ed medications
mexican pharmacy: Online Pharmacies in Mexico – mexican pharmacy
pet meds without vet prescription canada Prescription Drugs from Canada canadian family pharmacy
https://nolvadex.life/# tamoxifen buy
buy generic propecia without insurance cost of propecia tablets cheap propecia
https://finasteride.store/# buying cheap propecia prices
tamoxifen men: what is tamoxifen used for – tamoxifen and bone density
femara vs tamoxifen tamoxifen depression benefits of tamoxifen
https://cytotec.club/# order cytotec online
http://lisinopril.network/# lisinopril prinivil zestril
get cheap propecia without insurance: how cÉ‘n i get cheap propecia pills – get propecia without a prescription
https://nolvadex.life/# nolvadex vs clomid
tamoxifen for men nolvadex during cycle nolvadex price
http://ciprofloxacin.tech/# cipro ciprofloxacin
cost cheap propecia online propecia for sale cost cheap propecia for sale
cytotec pills buy online: buy cytotec in usa – cytotec pills buy online
https://finasteride.store/# generic propecia tablets
buy misoprostol over the counter: Misoprostol 200 mg buy online – buy misoprostol over the counter
tamoxifen and grapefruit tamoxifen reviews tamoxifen skin changes
http://finasteride.store/# cost of propecia without prescription
drug lisinopril: lipinpril – lisinopril 3972
buying cheap propecia without prescription buy cheap propecia online generic propecia
http://finasteride.store/# buy propecia pills
lisinopril 20mg discount: lisinopril 40 mg brand name – lisinopril 3.125
ciprofloxacin order online cipro pharmacy buy generic ciprofloxacin
http://finasteride.store/# cheap propecia
https://finasteride.store/# propecia tablet
ciprofloxacin generic price: buy cipro cheap – buy ciprofloxacin over the counter
ciprofloxacin 500 mg tablet price buy cipro online without prescription cipro pharmacy
http://cytotec.club/# buy cytotec online
tamoxifen and uterine thickening: tamoxifen side effects forum – tamoxifen blood clots
https://finasteride.store/# buy cheap propecia without rx
http://cytotec.club/# order cytotec online
buy cytotec pills online cheap buy cytotec over the counter cytotec online
tamoxifen headache: tamoxifen for gynecomastia reviews – tamoxifen and depression
buy cytotec online buy cytotec online cytotec online
https://ciprofloxacin.tech/# cipro ciprofloxacin
buy cytotec: cytotec online – buy cytotec over the counter
buy cialis pill buy cialis overseas Tadalafil Tablet
https://cialist.pro/# Generic Cialis price
http://cenforce.pro/# cenforce.pro
buy Levitra over the counter: Vardenafil online prescription – Cheap Levitra online
Levitra tablet price Vardenafil online prescription Levitra online USA fast
https://cialist.pro/# Generic Cialis without a doctor prescription
Kamagra 100mg: Kamagra tablets – Kamagra 100mg price
buy cenforce Cenforce 150 mg online Cenforce 100mg tablets for sale
http://levitrav.store/# buy Levitra over the counter
cheap viagra: Buy generic 100mg Viagra online – sildenafil online
Levitra 10 mg best price levitrav.store Cheap Levitra online
https://levitrav.store/# Levitra 10 mg buy online
Cenforce 100mg tablets for sale: cenforce for sale – cheapest cenforce
http://cialist.pro/# Cialis over the counter
Cialis 20mg price: Generic Tadalafil 20mg price – Tadalafil Tablet
Cheap Levitra online Cheap Levitra online Levitra generic best price
Sildenafil Citrate Tablets 100mg: Cheapest place to buy Viagra – Viagra without a doctor prescription Canada
http://kamagra.win/# Kamagra 100mg price
Purchase Cenforce Online cenforce.pro Cenforce 150 mg online
sildenafil oral jelly 100mg kamagra: buy kamagra online – Kamagra tablets
cenforce.pro cenforce for sale Cenforce 150 mg online
best online pharmacy no prescription online pharmacy best canadian pharmacy no prescription
http://pharmmexico.online/# reputable mexican pharmacies online
pharmacy website india: reputable indian online pharmacy – online pharmacy india
canada rx pharmacy world: trustworthy canadian pharmacy – safe canadian pharmacy
legal online pharmacy coupon code online pharmacy reputable online pharmacy no prescription
buying from online mexican pharmacy: mexican drugstore online – mexican drugstore online
http://pharmmexico.online/# pharmacies in mexico that ship to usa
how to get prescription drugs from canada: canada pharmacies online prescriptions – buying drugs without prescription
legal online pharmacy coupon code canadian pharmacy without prescription best no prescription pharmacy
https://pharmworld.store/# canadian online pharmacy no prescription
online pharmacy without prescription: cheapest pharmacy – pharmacy no prescription required
foreign pharmacy no prescription legal online pharmacy coupon code prescription drugs online
mexico pharmacies prescription drugs: mexican drugstore online – mexican mail order pharmacies
https://pharmcanada.shop/# prescription drugs canada buy online
buying prescription drugs in mexico: mexico drug stores pharmacies – mexican pharmaceuticals online
best online pharmacy india: indian pharmacies safe – п»їlegitimate online pharmacies india
pharmacy online no prescription buy medications without a prescription canadian prescription drugstore reviews
http://pharmnoprescription.icu/# ordering prescription drugs from canada
ordering drugs from canada: cheapest pharmacy canada – pharmacy canadian
http://pharmnoprescription.icu/# canadian pharmacy prescription
online pharmacy reviews no prescription no prescription needed online pharmacy quality prescription drugs canada
mexican online pharmacies prescription drugs: buying from online mexican pharmacy – mexican pharmaceuticals online
http://pharmnoprescription.icu/# order medication without prescription
online shopping pharmacy india: indian pharmacy online – mail order pharmacy india
doxycycline prices order doxycycline online doxycycline pills
how much is zithromax 250 mg: zithromax over the counter – zithromax prescription online
buy neurontin canada: neurontin capsules 600mg – neurontin prescription
https://doxycyclinea.online/# odering doxycycline
can you buy zithromax online how much is zithromax 250 mg zithromax 500 mg lowest price drugstore online
order amoxicillin no prescription: order amoxicillin online – antibiotic amoxicillin
prescription for amoxicillin: cost of amoxicillin prescription – antibiotic amoxicillin
neurontin 400mg: neurontin 400mg – neurontin 200 mg tablets
order doxycycline online: 100mg doxycycline – doxycycline 50 mg
https://doxycyclinea.online/# doxylin
cheap neurontin: neurontin tablets – neurontin 200 mg
amoxicillin medicine over the counter buy amoxicillin 500mg usa amoxicillin from canada
http://amoxila.pro/# can you buy amoxicillin over the counter
doxycycline generic: buy doxycycline online without prescription – doxycycline tablets
buy azithromycin zithromax zithromax 500 without prescription zithromax 600 mg tablets
50 mg prednisone canada pharmacy: prednisone 10 mg tablet – cheapest prednisone no prescription
https://amoxila.pro/# generic for amoxicillin
generic zithromax medicine zithromax tablets how to get zithromax
how to buy doxycycline online: odering doxycycline – where to purchase doxycycline
cheap zithromax pills: purchase zithromax z-pak – zithromax
neurontin online pharmacy neurontin singapore neurontin india
https://gabapentinneurontin.pro/# 32 neurontin
prednisone 40 mg price: prednisone online paypal – can i buy prednisone online in uk
neurontin 100mg tablet: generic neurontin – neurontin 100
prednisone 4mg tab prednisone canada prices order prednisone
https://zithromaxa.store/# zithromax 250 price
doxycycline: doxycycline 100mg capsules – cheap doxycycline online
order zithromax over the counter zithromax cost zithromax online usa
buy doxycycline online uk: doxycycline generic – doxy 200
https://zithromaxa.store/# zithromax for sale 500 mg
zithromax 250mg: can i buy zithromax over the counter – zithromax generic price
doxycycline 100mg capsules how to buy doxycycline online doxy
https://doxycyclinea.online/# doxy
buy cheap doxycycline: buy doxycycline 100mg – doxycycline hydrochloride 100mg
gabapentin medication: neurontin 100mg – neurontin 400 mg
zithromax 250 mg zithromax cost australia generic zithromax 500mg
how to order doxycycline: buy generic doxycycline – doxycycline pills
https://gabapentinneurontin.pro/# gabapentin 300mg
buy azithromycin zithromax: zithromax prescription in canada – how to buy zithromax online
cost of amoxicillin how to get amoxicillin over the counter amoxicillin online purchase
neurontin 300 mg: neurontin generic brand – brand name neurontin price
https://gabapentinneurontin.pro/# neurontin cost australia
neurontin 214 neurontin 200 neurontin 4000 mg
prednisone 10mg online: generic prednisone tablets – prednisone generic cost
zithromax 500mg price: zithromax online australia – how much is zithromax 250 mg
https://prednisoned.online/# prednisone 5084
prednisone tablets india buy prednisone online india buy generic prednisone online
zithromax capsules australia: where to get zithromax – zithromax 500 mg for sale
https://doxycyclinea.online/# doxycycline
prednisone pill: buy prednisone canada – prednisone where can i buy
cost of neurontin 100mg neurontin 4000 mg medicine neurontin capsules
buy doxycycline online: cheap doxycycline online – generic for doxycycline
http://amoxila.pro/# where can you get amoxicillin
doxy 200 doxycycline pills doxycycline without prescription
buy zithromax online australia: buy zithromax online cheap – zithromax 500 mg lowest price online
https://amoxila.pro/# amoxicillin from canada
5 mg prednisone tablets: prednisone best price – prednisone otc uk
order neurontin online buy neurontin neurontin 600 mg coupon
zithromax online usa: zithromax antibiotic – how to buy zithromax online
https://prednisoned.online/# generic over the counter prednisone
https://mexicanpharmacy1st.com/# medicine in mexico pharmacies
http://mexicanpharmacy1st.com/# best online pharmacies in mexico
mexican pharmacy mexican rx online reputable mexican pharmacies online
buying from online mexican pharmacy: buying prescription drugs in mexico – pharmacies in mexico that ship to usa
mexican online pharmacies prescription drugs: buying from online mexican pharmacy – medicine in mexico pharmacies
mexican pharmacy mexico drug stores pharmacies reputable mexican pharmacies online
https://mexicanpharmacy1st.com/# mexican drugstore online
mexican mail order pharmacies: mexican rx online – reputable mexican pharmacies online
https://mexicanpharmacy1st.com/# mexican pharmacy
mexican drugstore online reputable mexican pharmacies online medication from mexico pharmacy
https://mexicanpharmacy1st.shop/# mexican online pharmacies prescription drugs
mexican drugstore online: mexico pharmacy – best online pharmacies in mexico
medicine in mexico pharmacies: mexico pharmacies prescription drugs – pharmacies in mexico that ship to usa
https://mexicanpharmacy1st.com/# mexican mail order pharmacies
mexico drug stores pharmacies: buying from online mexican pharmacy – best mexican online pharmacies
https://mexicanpharmacy1st.shop/# medicine in mexico pharmacies
reputable mexican pharmacies online: mexican border pharmacies shipping to usa – medication from mexico pharmacy
mexican pharmaceuticals online mexican pharmacy mexico pharmacy
https://mexicanpharmacy1st.shop/# best online pharmacies in mexico
buying prescription drugs in mexico: best mexican online pharmacies – mexican online pharmacies prescription drugs
mexican mail order pharmacies: purple pharmacy mexico price list – mexican pharmaceuticals online
buying from online mexican pharmacy: mexican online pharmacies prescription drugs – purple pharmacy mexico price list
mexico drug stores pharmacies buying from online mexican pharmacy reputable mexican pharmacies online
https://mexicanpharmacy1st.com/# п»їbest mexican online pharmacies
mexico drug stores pharmacies: medication from mexico pharmacy – purple pharmacy mexico price list
https://cytotec.xyz/# buy cytotec online fast delivery
order cytotec online purchase cytotec cytotec buy online usa
get cheap propecia: propecia for sale – buying propecia tablets
cytotec online: Abortion pills online – buy cytotec pills online cheap
lisinopril 200mg lisinopril 20mg tablets cost lisinopril 10 mg tablets price
get cheap clomid pills: where can i get generic clomid no prescription – where can i get generic clomid
https://cytotec.xyz/# cytotec pills buy online
how to get generic clomid without dr prescription rx clomid can i get clomid online
buy cytotec: purchase cytotec – cytotec online
http://clomiphene.shop/# can you get cheap clomid without a prescription
zestril pill where to buy lisinopril 2.5 mg zestoretic 20 25
buy cytotec over the counter: buy cytotec online – buy cytotec in usa
https://lisinopril.club/# purchase lisinopril 10 mg
cheap clomid without insurance how to get cheap clomid without rx cost cheap clomid online
get cheap propecia tablets: propecia order – order propecia for sale
https://propeciaf.online/# buy propecia online
cytotec buy online usa: buy cytotec – buy cytotec in usa
neurontin 300 mg mexico: neurontin 300 – neurontin price uk
https://lisinopril.club/# lisinopril 49 mg
cytotec online Cytotec 200mcg price buy cytotec pills online cheap
lisinopril 25 mg price: lisinopril generic 20 mg – generic for prinivil
https://lisinopril.club/# buy lisinopril canada
buy cytotec cytotec online п»їcytotec pills online
order clomid no prescription: buying clomid without prescription – can i purchase clomid without rx
get clomid cost cheap clomid online where buy generic clomid no prescription
lisinopril prescription cost: lisinopril buy online – lisinopril 5mg pill
https://cheapestcanada.shop/# trustworthy canadian pharmacy
https://36and6health.com/# canadian pharmacy without prescription
http://36and6health.com/# best no prescription pharmacy
canadian pharmacy canadian pharmacy 1 internet online drugstore canadian pharmacy price checker
https://36and6health.com/# canadian pharmacy world coupon code
https://36and6health.shop/# no prescription pharmacy paypal
https://cheapestcanada.com/# reliable canadian pharmacy
canada rx pharmacy world cheapestcanada.com northwest canadian pharmacy
https://cheapestmexico.com/# best online pharmacies in mexico
https://36and6health.com/# cheapest pharmacy for prescriptions without insurance
canadian pharmacy 24 com: cheapestcanada.com – canadian pharmacy tampa
http://36and6health.com/# canadian pharmacy world coupon code
http://cheapestcanada.com/# vipps canadian pharmacy
https://cheapestandfast.shop/# canada drugs no prescription
cheap prescription drugs online cheapest & fast pharmacy canada prescription
https://cheapestindia.shop/# india online pharmacy
pharmacie en ligne france livraison internationale: pharmacies en ligne certifi̩es Рpharmacie en ligne france livraison internationale
farmacia online barata farmacias direct farmacias online baratas
migliori farmacie online 2024: п»їFarmacia online migliore – farmacia online senza ricetta
http://euapothekeohnerezept.com/# online apotheke
farmacias online seguras: farmacia en casa online descuento – farmacias online baratas
Pharmacie sans ordonnance: п»їpharmacie en ligne france – Pharmacie Internationale en ligne
pharmacie en ligne fiable pharmacie en ligne pharmacie en ligne livraison europe
acquistare farmaci senza ricetta: farmacia online senza ricetta – farmacia online
pharmacie en ligne france livraison belgique: Pharmacie en ligne livraison Europe Рacheter m̩dicament en ligne sans ordonnance
gГјnstigste online apotheke online apotheke preisvergleich online apotheke versandkostenfrei
pharmacie en ligne livraison europe: pharmacies en ligne certifiГ©es – pharmacie en ligne pas cher
farmacia barata: farmacia online envГo gratis – farmacia online madrid
farmacia online envÃo gratis: farmacia online madrid – farmacias online baratas
vardenafil hcl 10 mg
Farmacie online sicure farmaci senza ricetta elenco Farmacia online miglior prezzo
https://euapothekeohnerezept.shop/# online apotheke rezept
п»їFarmacia online migliore: comprare farmaci online con ricetta – acquisto farmaci con ricetta
farmacia online barcelona farmacia online madrid farmacia online espaГ±a envГo internacional
online apotheke preisvergleich: online apotheke deutschland – medikament ohne rezept notfall
farmacias online seguras: farmacias online seguras en espaГ±a – farmacias online seguras
ohne rezept apotheke: internet apotheke – günstigste online apotheke
farmacia online envГo gratis farmacia barata farmacia online barata
farmacias online baratas: farmacia online barata y fiable – farmacias online seguras en espaГ±a
pharmacie en ligne france pas cher: pharmacie en ligne – pharmacie en ligne france livraison internationale
https://eufarmacieonline.shop/# farmacia online
pharmacie en ligne livraison europe: pharmacie en ligne pas cher – pharmacie en ligne avec ordonnance
farmacias online seguras farmacia online barcelona farmacias direct
farmacia online barata y fiable: п»їfarmacia online espaГ±a – farmacia online barata
farmacia online barata y fiable: farmacia online envГo gratis – farmacias online seguras
Farmacie on line spedizione gratuita: comprare farmaci online all’estero – acquisto farmaci con ricetta
pharmacie en ligne france livraison belgique acheter mГ©dicament en ligne sans ordonnance п»їpharmacie en ligne france
farmacia online senza ricetta: Farmacia online miglior prezzo – Farmacie online sicure
farmacias online seguras en espa̱a: farmacias online seguras Рfarmacias online baratas
п»їshop apotheke gutschein apotheke online online apotheke gГјnstig
gГјnstige online apotheke: internet apotheke – online apotheke deutschland
http://eufarmaciaonline.com/# farmacia online madrid
medikament ohne rezept notfall: europa apotheke – medikament ohne rezept notfall
Farmacie on line spedizione gratuita: farmacie online affidabili – acquisto farmaci con ricetta
farmacias direct: п»їfarmacia online espaГ±a – farmacias direct
farmacias direct farmacias online seguras en espaГ±a farmacia online envГo gratis
Sildenafil teva 100 mg sans ordonnance: Prix du Viagra 100mg en France – Viagra pas cher livraison rapide france
pharmacie en ligne fiable: achat kamagra – pharmacie en ligne france fiable
trouver un mГ©dicament en pharmacie kamagra 100mg prix pharmacie en ligne sans ordonnance
Viagra prix pharmacie paris: viagra sans ordonnance – SildГ©nafil Teva 100 mg acheter
https://levitraenligne.com/# Achat médicament en ligne fiable
Pharmacie sans ordonnance: kamagra livraison 24h – pharmacie en ligne france livraison belgique
pharmacie en ligne france pas cher: pharmacie en ligne – pharmacies en ligne certifiГ©es
pharmacie en ligne fiable: levitra generique – pharmacie en ligne france pas cher
pharmacie en ligne france fiable: pharmacie en ligne pas cher – pharmacie en ligne livraison europe
Achat mГ©dicament en ligne fiable: Levitra sans ordonnance 24h – Achat mГ©dicament en ligne fiable
Hello!
This post was created with XRumer 23 StrongAI.
Good luck 🙂
Acheter Sildenafil 100mg sans ordonnance: Viagra generique en pharmacie – Viagra sans ordonnance 24h Amazon
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne sans ordonnance – п»їpharmacie en ligne france
Viagra pas cher livraison rapide france: Viagra homme prix en pharmacie sans ordonnance – Viagra pas cher livraison rapide france
pharmacie en ligne livraison europe: levitra generique – Pharmacie en ligne livraison Europe
pharmacie en ligne livraison europe: Pharmacies en ligne certifiees – pharmacie en ligne pas cher
Acheter Sildenafil 100mg sans ordonnance: viagra sans ordonnance – Viagra pas cher paris
Viagra gГ©nГ©rique sans ordonnance en pharmacie: Acheter du Viagra sans ordonnance – SildГ©nafil 100 mg sans ordonnance
https://kamagraenligne.com/# Pharmacie Internationale en ligne
Hello.
This post was created with XRumer 23 StrongAI.
Good luck 🙂
pharmacie en ligne france pas cher: achat kamagra – pharmacies en ligne certifiГ©es
pharmacies en ligne certifiГ©es: Levitra sans ordonnance 24h – acheter mГ©dicament en ligne sans ordonnance
pharmacie en ligne sans ordonnance: cialis generique – Pharmacie sans ordonnance
pharmacie en ligne pas cher: achat kamagra – pharmacie en ligne avec ordonnance
п»їpharmacie en ligne france: levitra en ligne – pharmacie en ligne france livraison internationale
pharmacie en ligne fiable: trouver un mГ©dicament en pharmacie – Pharmacie sans ordonnance
п»їpharmacie en ligne france: Levitra acheter – pharmacie en ligne france livraison belgique
trouver un mГ©dicament en pharmacie: Acheter Cialis – pharmacie en ligne pas cher
pharmacie en ligne france pas cher: Levitra sans ordonnance 24h – acheter mГ©dicament en ligne sans ordonnance
pharmacie en ligne livraison europe: levitra generique prix en pharmacie – acheter mГ©dicament en ligne sans ordonnance
Pharmacie Internationale en ligne: Acheter Cialis – Pharmacie en ligne livraison Europe
pharmacie en ligne france livraison internationale: Acheter Cialis 20 mg pas cher – pharmacie en ligne france pas cher
Pharmacie Internationale en ligne: achat kamagra – vente de mГ©dicament en ligne
Viagra homme prix en pharmacie sans ordonnance: Viagra gГ©nГ©rique pas cher livraison rapide – Viagra homme prix en pharmacie sans ordonnance
pharmacie en ligne france pas cher: kamagra 100mg prix – Pharmacie sans ordonnance
pharmacie en ligne: pharmacie en ligne livraison europe – pharmacie en ligne pas cher
Pharmacie sans ordonnance: kamagra en ligne – Pharmacie en ligne livraison Europe
pharmacie en ligne france pas cher: pharmacie en ligne sans ordonnance – pharmacie en ligne france livraison belgique
pharmacie en ligne france fiable: cialis prix – Pharmacie Internationale en ligne
vente de mГ©dicament en ligne: cialis prix – acheter mГ©dicament en ligne sans ordonnance
Viagra 100mg prix: Viagra sans ordonnance 24h – Viagra pas cher inde
vente de mГ©dicament en ligne: pharmacie en ligne sans ordonnance – Pharmacie Internationale en ligne
п»їpharmacie en ligne france: vente de mГ©dicament en ligne – pharmacie en ligne livraison europe
Viagra pas cher livraison rapide france: Viagra sans ordonnance 24h – Viagra Pfizer sans ordonnance
pharmacie en ligne: pharmacie en ligne – п»їpharmacie en ligne france
pharmacie en ligne france livraison internationale: kamagra 100mg prix – pharmacie en ligne pas cher
Pharmacie sans ordonnance: levitra generique prix en pharmacie – trouver un mГ©dicament en pharmacie
https://viaenligne.shop/# Viagra sans ordonnance livraison 48h
pharmacie en ligne france fiable: Acheter Cialis 20 mg pas cher – pharmacie en ligne avec ordonnance
trouver un mГ©dicament en pharmacie: cialis generique – pharmacie en ligne pas cher
acheter mГ©dicament en ligne sans ordonnance: levitra en ligne – pharmacie en ligne sans ordonnance
Pharmacie sans ordonnance: Acheter Cialis 20 mg pas cher – pharmacies en ligne certifiГ©es
Viagra Pfizer sans ordonnance: Viagra generique en pharmacie – Viagra homme prix en pharmacie sans ordonnance
acheter mГ©dicament en ligne sans ordonnance: Acheter Cialis – Pharmacie Internationale en ligne
acheter mГ©dicament en ligne sans ordonnance: kamagra 100mg prix – pharmacie en ligne avec ordonnance
pharmacie en ligne: kamagra gel – Achat mГ©dicament en ligne fiable
Pharmacie Internationale en ligne: kamagra livraison 24h – pharmacie en ligne sans ordonnance
Клюев А.В. и лучшие статьи по психотерапии.
Платформа Dragon money предлагает современные азартные игры. Использование промокодов добавит выгоды к вашему игровому процессу. Каждый найдет здесь игры по своему вкусу, включая самых искушенных игроков. В этом сообществе можно найти множество бездепозитных промокодов.
Ready to experience the excitement of video poker? Join our poker online free no money and start playing today. Whether you’re a novice or a seasoned poker player, our video poker games offer something for everyone. Embrace the challenge, apply your strategy, and enjoy the thrill of winning.
Slightly off topic 🙂
It so happened that my sister found an interesting man here, and recently got married ^_^
(Moderator, don’t troll!!!)
Is there are handsome people here! 😉 I’m Maria, 26 years old.
I work as a model, successfull – I hope you do too! Although, if you are very good in bed, then you are out of the queue!)))
By the way, there was no sex for a long time, it is very difficult to find a decent one…
And no! I am not a prostitute! I prefer harmonious, warm and reliable relationships. I cook deliciously and not only 😉 I have a degree in marketing.
My photo:
___
Added
The photo is broken, sorry(((
Check out my blog where you’ll find lots of hot information about me:
https://smm-media.ru
Or write to me in telegram @Lolla_sm1_best ( start chat with your photo!!!)
Вы можете изменить свою жизнь.
ОПСУИМОЛОГ покажет вам, как это сделать.
Опсуимолог усвоил все, чему учит, на собственном горьком опыте: сначала испортив собственную жизнь и по необходимости открыв инструменты и исследования, которые изменили жизнь и привели его туда, где он есть сегодня.
Миссия Опсуимолога проста: поделиться проверенными инструментами, которые помогут вам создать лучшую жизнь. Загляните на сайт опсуимолога, и вы будете смеяться вместе, учиться и шаг за шагом создавать свою новую жизнь — свою лучшую жизнь.
Простые советы опсуимолога, подкрепленные исследованиями, изменили жизни множества людей, и теперь он раскрывает вам все свои трудные секреты, веселые неудачи и интересные истории, чтобы вы могли изменить свою.
Hi, after reading this amazing article i am also glad to share my familiarity here with colleagues.
купить диплом механика
https://ekonty.com/blogs/view/55825
купить диплом повара-кондитера
Купить Кокаин в Москве? Самый чистый Кокаин в Москве Купить
ССЫЛКА НА САЙТ- https://mephedrone.top
Москва Купить Мефедрон? Кристаллы МЕФ?
Где в Москве Купить Мефедрон? САЙТ – https://mephedrone.top/
We offer premium databases for Xrumer 23 ai Strong and GSA Search Engine Ranker. Detailed information can be found here https://dseo24.monster
5]बैलेनसेट -1 ए बिक्री: संतुलन और कंपन विश्लेषण के लिए आपका आदर्श उपकरण
4]डिवाइस विवरण:
बैलेनसेट -1 ए संतुलन और कंपन विश्लेषण के लिए एक दोहरे चैनल उपकरण है, जो क्रशर, पंखे, अनाज हारवेस्टर हेलिकॉप्टर, शाफ्ट, सेंट्रीफ्यूज, टर्बाइन, और बहुत कुछ जैसे रोटर्स को संतुलित करने के लिए आदर्श है ।
4]विशेषताएं:
3]वाइब्रोमीटर मोड:
टैकोमीटर: रोटेशन स्पीड (आरपीएम) को सटीक रूप से मापता है ।
चरण: सटीक विश्लेषण के लिए कंपन संकेतों के चरण कोण को निर्धारित करता है ।
1 एक्स कंपन: मुख्य आवृत्ति घटक को मापता है और विश्लेषण करता है ।
एफएफटी स्पेक्ट्रम: कंपन सिग्नल आवृत्ति स्पेक्ट्रम का विस्तृत दृश्य प्रदान करता है ।
समग्र कंपन: समग्र कंपन स्तरों को मापता है और मॉनिटर करता है ।
मापन लॉग: विश्लेषण के लिए माप डेटा बचाता है ।
3]संतुलन मोड:
एकल-विमान संतुलन: कंपन को कम करने के लिए एकल विमान में रोटार को संतुलित करता है ।
दो-विमान संतुलन: गतिशील संतुलन के लिए दो विमानों में रोटार को संतुलित करता है ।
ध्रुवीय आरेख: सटीक वजन प्लेसमेंट के लिए ध्रुवीय आरेख पर असंतुलन की कल्पना करता है ।
अंतिम सत्र पुनर्प्राप्ति: सुविधा के लिए पिछले संतुलन सत्र को फिर से शुरू करने की अनुमति देता है ।
सहिष्णुता कैलकुलेटर (आईएसओ 1940): आईएसओ 1940 मानकों के अनुसार स्वीकार्य असंतुलन मूल्यों की गणना करता है ।
पीस व्हील बैलेंसिंग: असंतुलन को खत्म करने के लिए तीन सुधार भार का उपयोग करता है ।
3]आरेख और रेखांकन:
सामान्य रेखांकन: समग्र कंपन का दृश्य।
1 एक्स रेखांकन: मुख्य आवृत्ति कंपन पैटर्न का प्रदर्शन।
हार्मोनिक रेखांकन: हार्मोनिक आवृत्तियों के प्रभाव को दर्शाता है ।
वर्णक्रमीय रेखांकन: गहन विश्लेषण के लिए आवृत्ति स्पेक्ट्रम का चित्रमय प्रतिनिधित्व ।
3]अतिरिक्त सुविधाएँ:
संग्रह: भंडारण और पिछले संतुलन सत्रों तक पहुंच ।
रिपोर्ट: संतुलन परिणामों पर विस्तृत रिपोर्ट तैयार करना ।
पुनर्संतुलन: सहेजे गए डेटा का उपयोग करके आसानी से संतुलन प्रक्रिया को दोहराएं ।
सीरियल प्रोडक्शन बैलेंसिंग: सीरियल प्रोडक्शन में रोटार को संतुलित करने के लिए उपयुक्त ।
इंपीरियल और मीट्रिक सिस्टम विकल्प: दुनिया भर में संगतता और सुविधा प्रदान करता है ।
4]कीमत:
€1751
3]पैकेज में शामिल हैं:
मापन इकाई
दो कंपन सेंसर
चुंबकीय स्टैंड के साथ ऑप्टिकल सेंसर (लेजर टैकोमीटर)
वजन
सॉफ्टवेयर (लैपटॉप शामिल नहीं है, एक अतिरिक्त आदेश के रूप में उपलब्ध है)
प्लास्टिक ले जाने का मामला
पोर्टेबल बैलेंसर और कंपन विश्लेषक खरीदें
आज ही बैलेनसेट -1 ए ऑर्डर करें और अपने रोटार का सटीक संतुलन सुनिश्चित करें!
Russische DJs liste
you’ll have hard on all night —> http://zi.ma/3kmnv4/
वित्तीय स्वतंत्रता की तलाश करने वाले सभी लोगों के लिए रोबोट सबसे अच्छा तरीका है । https://t.me/cryptaxbot/11
диплом государственного образца купить диплом государственного образца купить .
Pin up 306 casino: Pin up 306 casino – Pin Up
https://autolux-azerbaijan.com/# Pin Up Azerbaycan ?Onlayn Kazino
Pin Up Kazino ?Onlayn: Pin-up Giris – Pin-Up Casino
Rybelsus – Quick and Easy Weight Lass
According to randomised controlled trials, you start losing weight immediately after taking Rybelsus. After one month, the average weight loss on Rybelsus is around 2kg; after two months, it’s over 3kg.
What does Rybelsus do to your body?
Rybelsus (oral semaglutide) is a GLP-1 receptor agonist. It mimics a fullness hormone called GLP-1.
Rybelsus reduces appetite and hunger by interacting with the brain’s appetite control centre, the hypothalamus. This effect on the brain helps you eat fewer calories and starts almost immediately after taking the pill.
However, you might notice your hunger levels rising and falling in the first 4-5 weeks you take Rybelsus.
It can take around 4-5 weeks for Rybelsus to reach a level in the body we call a steady state. A steady state is when the drug’s levels in the body remain consistent rather than spiking and falling.
Interestingly, this initial weight loss is no different to other weight loss treatments or the impact of diet interventions on weight loss. The real effect of oral semaglutide is seen beyond three months.
Oral semaglutide is a long-acting medication that’s started at a lower dose to reduce the number and severity of side effects as it’s built up to a higher maintenance dose.
https://true-pill.top/rybelsus.html
https://autolux-azerbaijan.com/# pin-up kazino
pin-up360: Pin Up Azerbaycan – pin-up kazino
?Onlayn Kazino: pin-up kazino – Pin Up
Pin up 306 casino: Pin-Up Casino – Pin-Up Casino
https://autolux-azerbaijan.com/# pin-up360
pin-up 141 casino: pin-up360 – pin-up kazino
https://autolux-azerbaijan.com/# ?Onlayn Kazino
Получите еще больше удовольствия от игры в up-x с нашими уникальными промокодами. Загляните на up-x промокоды и получите свой бонус прямо сейчас.
bespoke metal screen https://dk-construction.com.au/screen-fences
Don’t wait any longer to experience the thrill of online slots. Join our online slots casino report today and embark on an adventure filled with fun, excitement, and endless opportunities to win. With our wide selection of games, generous bonuses, and secure environment, there’s no better place to play. Sign up now and start spinning the reels for your chance to hit the jackpot!
अब खेल में नए टोकन प्राप्त करें Hamster Kombat
दैनिक वितरण आपके पर्स में नहीं
हमारी परियोजना में शामिल हों notreward.pro और टोनकोइन प्राप्त करें
हैमस्टरकोम्बैट इनाम
इस कार्यक्रम के साथ अपने लैपटॉप को एक वित्तीय साधन बनाएं । Telegram – @cryptaxbot
good morning images new https://t.me/good_morning_images_new
Old man with young wife. Donald Trump Approves —> http://zi.ma/30ywvb/
Cat casino регулярно предлагает своим игрокам новые промокоды, которые позволяют получить бонусы и увеличить свои выигрыши. Не пропустите эти выгодные предложения.
Используйте промокоды Cat Casino при пополнении счета, чтобы получить больше бонусных средств. Эти коды доступны для всех игроков и регулярно обновляются на сайте.
सेवानिवृत्त लोगों के लिए सबसे अच्छा ऑनलाइन नौकरी । अपने बुढ़ापे को अमीर बनाओ। https://t.me/cryptaxbot/14
MOBILE GAMES & APP DEVELOPMENT
https://www.fanstudio.co.uk/
इस बॉट का उपयोग करके इंटरनेट में पैसा कमाएं । यह वास्तव में काम करता है!] https://t.me/cryptaxbot/14?untontoub53gep
वित्तीय रोबोट पैसा बनाने का आपका # 1 विशेषज्ञ है । ] https://t.me/cryptaxbot/14?untontoub24gep
वित्तीय स्वतंत्रता की तलाश करने वाले सभी लोगों के लिए रोबोट सबसे अच्छा तरीका है । https://t.me/cryptaxbot/14?untontoub29gep
Играйте бесплатно в популярные автоматы без регистрации и смс на нашем сайте – увлекательное времяпрепровождение и возможность испытать удачу в азартных играх.
smartblip home gadgets https://smartblip.com best price
पूरे दिन कमाई रखने के लिए स्वचालित रोबोट को आज़माएं । ] https://t.me/cryptaxbot/17?untontoub56gep
Промокоды Vavada казино помогут вам получить дополнительные средства для игры. Воспользуйтесь бонусами на депозиты и фриспинами, чтобы увеличить свои шансы на победу.
एक बच्चा भी पैसा कमाना जानता है । यह रोबोट आपको क्या चाहिए! https://t.me/cryptaxbot/17?untontoub32gep
एक डॉलर कुछ भी नहीं है, लेकिन यह यहां $100 में बढ़ सकता है । ] https://t.me/cryptaxbot/17?untontoub81gep
Воспользуйтесь промокодами Vavada казино, чтобы получить бонусы на депозиты и фриспины. Эти предложения сделают вашу игру более выгодной и интересной.
Blackjack is not just a game of luck; it’s a thrilling challenge that can be mastered with the right strategy. Imagine the excitement of hitting 21 and beating the dealer with confidence. Whether you’re a novice or an experienced player, learning blackjack chart can transform your gaming experience and lead to significant wins. Don’t miss out on the opportunity to improve your skills and enjoy the rewards. Start playing blackjack today and take the first step towards becoming a pro!
Воспользуйтесь промокодами Drip казино, чтобы получить дополнительные бонусы. Они помогут увеличить ваш игровой баланс и сделать процесс игры более интересным. Drip казино промокоды откроют новые возможности.
ऑनलाइन वित्तीय रोबोट आपकी सफलता की कुंजी है । https://t.me/cryptaxbot/17?untontoub81gep
Промокоды Vavada казино открывают новые возможности для получения бонусов на депозиты и фриспины, что делает игровой процесс более захватывающим и выгодным. Vavada казино бонусы помогут вам выиграть.
Промокоды Drip казино открывают новые возможности для игроков, предоставляя дополнительные бонусы и фриспины. Drip казино бонусы сделают вашу игру лучше.
Использование промокодов Vavada казино помогает игрокам наслаждаться дополнительными бонусами и фриспинами, делая игровой процесс более интересным. Vavada казино бонусы увеличивают игровой баланс.
Roulette is more than just a game of chance; it’s a captivating experience that offers endless possibilities. Imagine the rush of placing your bet on red or black and watching the wheel spin in anticipation. Whether you’re a beginner or a seasoned player, roulette promises excitement and the chance to win big. Embrace the challenge, learn the strategies, and enjoy the thrill of this iconic casino game. Start your journey with online roulette simulator today and let the excitement of the wheel propel you towards unforgettable wins!
रोबोट लॉन्च होने पर अब और काम करने की आवश्यकता नहीं है!] https://t.me/cryptaxbot/17?untontoub74gep
Step into the thrilling world of gambling, where every card you draw could be the key to victory! Whether you’re a seasoned player or a newcomer, our online casinos offer endless excitement and the chance to master your skills. Join us now and experience the ultimate casinos list adventure!
https://e-porn.net free xxx tube videos webcams dating,
онлайн камеры, знакомства
Explore Catizen, the game where gaming pays off! Raise adorable cats, finish tasks, and trade items. Collect rewards, cash out, and have fun! Join now: https://t.me/catizenbot/gameapp?startapp=r_752_15277420
Используйте промокоды Vavada казино для получения эксклюзивных бонусов. Дополнительные фриспины и депозитные бонусы ждут вас.
Drip казино промокоды помогут вам получить дополнительные бонусы и фриспины, увеличивая шансы на выигрыш. Наслаждайтесь игрой с дополнительными возможностями.
Промокоды Vavada казино позволяют увеличить депозит и получить дополнительные фриспины. Эти преимущества делают вашу игру еще более захватывающей и прибыльной.
प्रयासों के बिना अतिरिक्त पैसा कमाएं । https://t.me/cryptaxbot/17?untontoub97gep
Активируйте Vavada казино промокоды и получите доступ к эксклюзивным бонусам. Наслаждайтесь игрой с дополнительными привилегиями и предложениями от Vavada.
Получите максимум удовольствия с промокодами Drip казино. Откройте для себя уникальные бонусы и привилегии, доступные только по специальным промокодам Drip казино.
Активируйте Vavada казино промокоды и получите эксклюзивные бонусы. Наслаждайтесь игрой с дополнительными привилегиями, доступными только по специальным промокодам от Vavada казино.
Промокоды Vavada казино – это ваш шанс на дополнительные бонусы. Узнайте, как получить и использовать промокоды для увеличения вашего игрового опыта в Vavada казино.
Откройте для себя мир бонусов с промокодами Drip казино. Получите дополнительные привилегии и наслаждайтесь игрой с уникальными предложениями от Drip казино.
Vavada казино предлагает уникальные промокоды для своих игроков. Получите дополнительные бонусы и привилегии, активировав промокоды на сайте Vavada казино.
Промокоды Vavada казино – это ваш путь к дополнительным бонусам. Активируйте промокод и наслаждайтесь игрой с эксклюзивными предложениями от Vavada казино.
Используйте Drip казино промокоды для получения дополнительных бонусов. Наслаждайтесь игрой с уникальными предложениями и привилегиями от Drip казино.
Промокоды Vavada казино – ваш ключ к уникальным бонусам и привилегиям. Узнайте, как активировать промокод и получите больше удовольствия от игры в Vavada казино.
Промокоды Vavada казино – это ваш шанс на дополнительные бонусы. Узнайте, как получить и использовать промокоды для увеличения вашего игрового опыта в Vavada казино.
Промокоды Drip казино открывают доступ к эксклюзивным бонусам и предложениям. Узнайте, как активировать промокод и получите больше от вашей игры в Drip казино.
Активируйте промокоды Vavada казино и наслаждайтесь дополнительными привилегиями. Эти промокоды открывают доступ к уникальным предложениям и бонусам.
Промокоды Vavada казино предлагают уникальные бонусы и привилегии. Используйте Vavada казино промокоды для получения дополнительных преимуществ в игре.
Vavada казино предлагает эксклюзивные промокоды для своих игроков. Активируйте эти промокоды и получите дополнительные бонусы и привилегии.
Ищете лучшие промокоды для Drip казино? Используйте Drip казино промокоды для получения эксклюзивных бонусов и наслаждайтесь игрой.
купить диплом университета ast-diplom.com .
купить диплом пту в красноярске ast-diplomy.com .
Привет, друзья!
Где заказать диплом по актуальной специальности?
Приобрести диплом университета.
http://kingcityrp.moibb.ru/posting.php?mode=post&f=34
Хорошей учебы!
“Собачий Дом (doghouse)” — это признанный слот от компании Pragmatic Play, лидера в индустрии азартных игр, который обеспечивает захватывающий ход игры на пяти барабанах и двадцати призовых линиях.
Любители азарта переносятся в захватывающую вселенную очаровательных собак, где каждый спин может принести большие выигрыши.
Рассматриваемый видеослот привлекает внимание своей насыщенной и завораживающей графикой, а также различными дополнительными возможностями.
Особого внимания заслуживают — вайлды, подменяющие другие изображения для создания оплачиваемых последовательностей, мультипликаторы, существенно увеличивающие выигрыши, и бонусные вращения, которые можно активировать при появлении трех или более скаттеров.
В целом, этот увлекательный слот манит игроков неповторимыми веселыми персонажами и потенциалом добиться внушительных результатов.
Looking for a thrilling gaming experience without spending a dime? Discover the excitement of a free casino games, where you can enjoy your favorite games and win big without risking your hard-earned money!
Step into the thrilling world of online casino, where every card you draw could be the key to victory! Whether you’re a seasoned player or a newcomer, our online casinos offer endless excitement and the chance to master your skills. Join us now and experience the ultimate casinos list adventure!
Привет!
Где приобрести диплом специалиста?
Заказать диплом университета.
http://forum.analysisclub.ru/index.php/topic,190201.new.html#new
Хорошей учебы!
Привет!
Купить диплом о высшем образовании
Наши специалисты предлагают выгодно заказать диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями. Документ пройдет лубую проверку, даже при помощи специальных приборов. Решите свои задачи быстро с нашей компанией.
Где заказать диплом по актуальной специальности?
https://1abakan.ru/forum/showthread-218496/
Будем рады вам помочь!.
Good manners go a long way. Being punctual, polite, and respectful during your date sets a positive tone and shows you value their time
news site
купить диплом электромонтажника asxdiplomik24.ru .
Здравствуйте!
Задумался а действительно можно купить диплом государственного образца в Москве, и был удивлен, все реально и главное официально!
Сначала серфил в сети и искал такие темы как: купить диплом в соликамске, купить диплом для иностранцев, купить диплом в верхней пышме, купить диплом в ангарске, купить диплом в волгодонске, получил базовую информацию.
Остановился в итоге на материале купить диплом специалиста, https://drc.uog.edu.et/presentation-2015
Хорошей учебы!
Notice any red flags? Trust your gut. If something feels off, it’s okay to step back and reassess the situation. Your safety and comfort come first
news site
Промокоды Vavada казино предоставляют отличные бонусы для всех игроков. Найдите актуальные коды и используйте их для получения дополнительных привилегий.
Drip казино предлагает уникальные бонусы для своих игроков с использованием промокодов. Найдите свежие коды и наслаждайтесь всеми преимуществами.
Найдите актуальные промокоды Vavada казино и получите дополнительные бонусы для игры. Эти коды предлагают отличные возможности для всех любителей азартных игр.
Официальная покупка диплома вуза с сокращенной программой обучения в Москве
Привет, друзья!
Купить диплом ВУЗа
Мы предлагаем выгодно и быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Наш документ способен пройти любые проверки, даже при использовании профессионального оборудования. Решите свои задачи быстро и просто с нашими дипломами.
Где заказать диплом по нужной специальности?
Всё о покупке аттестата о среднем образовании: полезные советы
купить диплом в иваново asxdiplomik.com/kupit-diplom-moskva .
Как купить аттестат 11 класса с официальным упрощенным обучением в Москве
appleincub.ru/kupit-diplom-ekspress-dostavka
Срочно Требуются: Курьеры-регистраторы
Ищем активных граждан РФ, живущих в Москве и области!
А так же, принимаются люди, для работы, проживающие в других регионах рф,
кроме (северного Кавказа)
Заработок от 3000 тыс. до 7000 тыс. за каждый выезд.
Совмещай работу с другими делами – гибкий график!
Твоя задача – регистрация компаний. Просто и выгодно!
Оплата сразу после выполнения задания!
Подходишь по возрасту (18-60 лет)Присоединяйся!
Начни зарабатывать больше прямо сейчас – ждем именно тебя!
А так же, требуются люди, на удаленную работу, по поиску и подбору
директоров (Курьеров-регистраторов) зп, от 5000 тысяч рублей, за подобранного человека.
#работа #вакансия #Москва #курьер #регистратор #заработок #график #подработка
Начни зарабатывать больше прямо сейчас – ждем именно тебя!
https://chat.whatsapp.com/InLwqrVeXucCbCTqxkwrSo
Как получить диплом стоматолога быстро и официально
earthpro.listbb.ru/viewtopic.php?f=3&t=396
Slightly off topic 🙂
It so happened that my sister found an interesting man here, and recently got married ^_^
(Admin, don’t troll!!!)
Is there are handsome people here! 😉 I’m Maria, 25 years old.
I work as a model, successfull – I hope you do too! Although, if you are very good in bed, then you are out of the queue!)))
By the way, there was no sex for a long time, it is very difficult to find a decent one…
And no! I am not a prostitute! I prefer harmonious, warm and reliable relationships. I cook deliciously and not only 😉 I have a degree in marketing.
My photo:
___
Added
The photo is broken, sorry(((
Check out my blog where you’ll find lots of hot information about me:
https://web-rnd.ru
Or write to me in telegram @Lolla_sm1_best ( start chat with your photo!!!)
Hello!
Do you want to become the best SEO specialist and link builder or do you want to outpace your competitors?
Premium base for XRumer
$119/one-time
Get access to our premium database, which is updated monthly! The database contains only those resources from which you will receive active links – from profiles and postings, as well as a huge collection of contact forms. Free database updates. There is also the possibility of a one-time purchase, without updating the databases, for $38.
Fresh base for XRumer
$94/one-time
Get access to our fresh database, updated monthly! The database includes active links from forums, guest books, blogs, etc., as well as profiles and activations. Free database updates. There is also the possibility of a one-time purchase, without updating the databases, for $25.
GSA Search Engine Ranker fresh verified link list
$119/one-time
Get access to our fresh database, updated monthly! The fresh database includes verified and identified links, divided by engine. Free database updates. There is also the possibility of a one-time purchase, without updating the databases, for $38.
GSA Search Engine Ranker activation key
$65
With GSA Search Engine Ranker, you’ll never have to worry about backlinks again. The software creates backlinks for you 24 hours a day, 7 days a week. By purchasing GSA Search Engine Ranker from us, you get a quality product at a competitive price, saving your resources.
To contact us, write to telegram https://t.me/DropDeadStudio
Промокоды Vavada казино предлагают уникальные бонусы. Найдите свежие коды и улучшите свои игровые впечатления с этими привилегиями.
Найдите актуальные промокоды Drip казино и получите дополнительные привилегии. Эти коды предоставляют уникальные бонусы для всех игроков.
Промокоды Vavada казино предоставляют уникальные бонусы для всех игроков. Воспользуйтесь этими кодами и наслаждайтесь всеми привилегиями.
купить диплом нефтяного asxdiplomik.com/kupit-diplom-moskva .
Полезная информация как купить диплом о высшем образовании без рисков
ast-diploms.com/kupit-diplom-moskva
купить диплом с оценками купить диплом с оценками .
аттестаты купить гознак diplomyx.com .
купить диплом о среднем образовании в липецке diplomasx.com .
купить аттестаты за 11 ast-diploms.com .
Привет, друзья!
Мы можем предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Вы сможете заказать качественный диплом за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы и аттестаты печатаются на “правильной” бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. Они заверяются необходимыми печатями и штампами.
Заказать диплом любого университета.
stareishina.com/gallery/image/3645-russiany-diplomanscom/
Хорошей учебы!
Привет, друзья!
Мы предлагаем дипломы любой профессии по приятным ценам. Цена зависит от определенной специальности, года выпуска и образовательного учреждения.
Где приобрести диплом специалиста?
Приобрести диплом ВУЗа
landik-diploms-srednee.ru/kupit-diplom-volgograd
Хорошей учебы!
Добрый день!
Заказать документ о получении высшего образования вы имеете возможность в нашей компании в столице.
asxdiplomik24.ru/kupit-diplom-ekaterinbur
Хорошей учебы!
Промокоды Vavada казино открывают доступ к уникальным бонусам. Воспользуйтесь актуальными предложениями и наслаждайтесь всеми преимуществами игры.
Воспользуйтесь промокодами Drip казино для получения дополнительных привилегий. Эти коды обеспечат вам лучшие условия для игры.
Промокоды Vavada казино помогут вам увеличить игровой баланс. Найдите актуальные коды и наслаждайтесь дополнительными привилегиями.
Привет, друзья!
Полезные советы по покупке диплома о высшем образовании без риска
kolhos.listbb.ru/viewtopic.php?f=2&t=605
Будем рады вам помочь!.
Добрый день!
Приобрести документ ВУЗа вы сможете в нашем сервисе. Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов Российской Федерации. Вы получите диплом по любым специальностям, включая документы старого образца.
avtoweek2016.ru/kupite-diplom-i-dostignite-novyih-vyisot/
Хорошей учебы!
Здравствуйте!
Где приобрести диплом специалиста?
Приобрести диплом любого университета.
iqtorg.ru/forum/messages/forum2/topic629/message703/?result=new#message703
Привет, друзья!
Приобрести диплом о высшем образовании.
flerus-shop.hcp.dilhost.ru/club/user/3/forum/message/876/872/
школьный аттестат купить http://www.diploms-x.com/ .
Добрый день!
Мы готовы предложить документы техникумов, расположенных в любом регионе Российской Федерации. Можно купить диплом от любого учебного заведения, за любой год, включая документы старого образца. Документы выпускаются на бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Документы будут заверены необходимыми печатями и подписями.
mormoniconoclast.net/deadpool-movie-review
Здравствуйте!
Где заказать диплом специалиста?
Мы предлагаем документы институтов, расположенных в любом регионе РФ. Вы имеете возможность купить качественно сделанный диплом за любой год, включая документы СССР. Дипломы выпускаются на бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригинала. Документы заверяются необходимыми печатями и штампами.
Мы изготавливаем дипломы любых профессий по приятным тарифам.
diplomyx.com/kupit-diplom-magistra
Всегда вам поможем!
Привет!
Где заказать диплом по нужной специальности?
tatuheart.ukrbb.net/posting.php?mode=post&f=41&sid=7b3ba5ef4a427c7dadb8d2bc2904d87c
Удачи!
командир купил диплом asxdiplomik24.ru .
Добрый день!
Наши специалисты предлагают быстро и выгодно купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Наш документ способен пройти любые проверки, даже при использовании профессиональных приборов. Решите свои задачи быстро и просто с нашим сервисом.
artshi.ru/index.php?subaction=userinfo&user=apypyx
Хорошей учебы!
Добрый день!
Где приобрести диплом специалиста?
Мы изготавливаем дипломы любой профессии по приятным тарифам.
arahn.100webspace.net/profile.php?mode=viewprofile&u=142536
Привет!
Диплом вуза купить официально с упрощенным обучением в Москве
bandit400.ru/index.php?subaction=userinfo&user=ujohiqy
Рады оказаться полезными!.
Добрый день!
Приобрести диплом о высшем образовании.
school97.ru/vesti/view_profile.php?UID=208294
Успешной учебы!
Looking for a quick financial boost? Apply now for a small personal installment loans and secure the funds you need with ease. Whether it’s for unexpected expenses, home improvements, or a special purchase, our fast approval process ensures you get the money swiftly. Don’t wait – take the first step towards financial freedom today!
Здравствуйте!
Купить документ о получении высшего образования можно в нашем сервисе.
ast-diplomy24.ru/kupit-diplom-nizhnij-novgorod
Хорошей учебы!
Здравствуйте!
Заказать документ ВУЗа можно у нас в столице.
diploms-x24.ru/kupit-diplom-vracha
Здравствуйте!
Можно ли купить аттестат о среднем образовании, основные моменты и вопросы
afterpad.com/forums/post.php?fid=7
Рады оказаться полезными!.
можно ли купить образование можно ли купить образование .
врач с купленным дипломом diplomyx.com .
Добрый день!
Мы предлагаем дипломы любой профессии по приятным тарифам. Стоимость будет зависеть от определенной специальности, года получения и университета.
landik-diploms-srednee.ru/attestat-shkoly
Успехов в учебе!
Воспользуйтесь промокодами Vavada казино для получения эксклюзивных бонусов. Эти коды предоставляют отличные возможности для всех любителей азартных игр.
Используйте промокоды Drip казино для получения дополнительных бонусов. Эти коды предоставляют отличные возможности для всех игроков.
Найдите свежие промокоды Vavada казино и получите дополнительные бонусы для игры. Эти коды помогут сделать ваше время в казино еще более захватывающим и интересным.
Привет!
Мы изготавливаем дипломы любой профессии по приятным ценам.
anglais.ru/category/other/commonmistakes/index.html
Привет, друзья!
Приобрести документ о получении высшего образования можно у нас в столице.
diplomyx24.ru/kupit-diplom-krasnoyarsk
Хорошей учебы!
Сколько стоит диплом высшего и среднего образования и как его получить?
http://www.hebergementweb.org/threads/kupit-diplom-s-garantiej-podlinnosti.1480408/
Привет!
Легальные способы покупки диплома о среднем полном образовании
graph.org/Oficialnyj-diplom-bez-ucheby-i-ehkzamenov-07-12
Рады оказаться полезными!.
Привет, друзья!
Узнайте стоимость диплома высшего и среднего образования и процесс получения
landik-diploms-srednee.ru/svidetelstvo-o-rozhdenii
Успешной учебы!
Добрый день!
Где купить диплом специалиста?
Приобрести диплом любого университета.
app-s.ru/blog/kak_podyskat_nadezhnyj_magazin_s_ogromnym_katalogom_diplomov/2024-07-04-1868
become our affiliate
https://affiliates.whalehunter.cash/track/Kirill.18AffRef.18AffRef.MAIN.0.0.0.0.0.0.0.0
Привет!
Где приобрести диплом специалиста?
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по доступным ценам. Стоимость может зависеть от выбранной специальности, года выпуска и образовательного учреждения. Стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас очень важно, чтобы дипломы были доступными для большого количества граждан.
arusak-diploms-srednee.ru
Успешной учебы!
Привет!
Где купить диплом специалиста?
Купить диплом любого университета.
rst.adk.audio/company/personal/user/1577/forum/message/1952/1984/#message1984
Здравствуйте!
Приобрести документ университета можно в нашей компании в Москве.
diplomasx24.ru/kupit-diplom-nizhnij-novgorod
Промокоды Vavada казино открывают доступ к уникальным бонусам и привилегиям. Эти коды помогут вам улучшить свои игровые впечатления и получить больше удовольствия от игры.
Промокоды Drip казино предлагают эксклюзивные бонусы для всех игроков. Эти коды делают игру более захватывающей и интересной.
Используйте промокоды Vavada казино для получения эксклюзивных бонусов. Эти коды обеспечивают доступ к уникальным привилегиям и улучшенным условиям игры.
Быстрое обучение и получение диплома магистра – возможно ли это?
ast-diplomas.com/kupit-diplom-nizhnij-novgorod
Привет!
Готовы предложить документы техникумов, которые находятся на территории всей Российской Федерации. Вы сможете заказать диплом за любой год, включая документы старого образца. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригиналов. Документы будут заверены всеми необходимыми печатями и подписями.
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Цена зависит от конкретной специальности, года получения и университета. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы документы были доступными для подавляющей массы граждан.
landik-diploms-srednee.ru/kupit-diplom-saratov
Удачи!
Привет!
Заказать документ о получении высшего образования вы имеете возможность у нас в Москве.
ast-diploms24.ru/kupit-diplom-voronezh
Успехов в учебе!
Информация о букмекерской конторе Mostbet, особенностях ставок и возможностях для игроков.
купить диплом делопроизводитель ast-diploms.com .
Здравствуйте!
Где приобрести диплом по нужной специальности?
Мы готовы предложить дипломы любой профессии по приятным ценам. Цена будет зависеть от определенной специальности, года выпуска и образовательного учреждения. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступны для большинства наших граждан.
Для вас можем предложить дипломы психологов, юристов, экономистов и любых других профессий по разумным ценам.
toplentanews.ru/kupit-diplom-rf-v-moskve-ofitsialnoe-oformlenie-i-garantii
Окажем помощь!.
купить диплом сертификат купить диплом сертификат .
Привет!
Где приобрести диплом по необходимой специальности?
Мы предлагаем документы ВУЗов, расположенных на территории всей Российской Федерации. Можно купить качественный диплом за любой год, включая сюда документы СССР. Дипломы печатаются на “правильной” бумаге высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. Они будут заверены необходимыми печатями и штампами.
Мы изготавливаем дипломы любой профессии по приятным ценам.
ast-diploms24.ru/kupit-diplom-o-srednem-obrazovanii
Рады оказаться полезными!
Привет, друзья!
Процесс получения диплома стоматолога: реально ли это сделать быстро?
ilovehabbo.bbon.ru/viewtopic.php?id=1558#p28974
Рады оказать помощь!.
Привет!
Мы готовы предложить документы техникумов, которые находятся в любом регионе России. Вы сможете заказать качественный диплом от любого заведения, за любой год, указав необходимую специальность и оценки за все дисциплины. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригиналов. Документы заверяются всеми обязательными печатями и подписями.
saopaulofansclub.com/read-blog/2794
Здравствуйте!
Наши специалисты предлагают максимально быстро заказать диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями должностных лиц. Наш диплом способен пройти лубую проверку, даже с применением специальных приборов. Достигайте свои цели максимально быстро с нашим сервисом.
moskovskij.getbb.ru/viewtopic.php?f=12&t=2231
Успешной учебы!
где можно купить диплом о среднем asxdiplomik24.ru .
Привет!
Где купить диплом по необходимой специальности?
cod4.flybb.ru/viewtopic.php?f=60&t=655&sid=94b62751a76127472ede4c2162f410da
Успехов в учебе!
Здравствуйте!
Быстрое обучение и получение диплома магистра – возможно ли это?
aromatov.wooden-rock.ru/forum/topic.php?forum=1&topic=15825&v=l#1719302971
Всегда вам поможем!.
Привет!
Приобрести диплом ВУЗа.
vipka.0bb.ru/viewtopic.php?id=5050#p7735
Успехов в учебе!
вуз инфо дипломы отзывы [url=http://www.diploms-x.com]вуз инфо дипломы отзывы[/url] .
Привет, друзья!
Готовы предложить документы ВУЗов, которые расположены в любом регионе России. Можно приобрести качественно напечатанный диплом за любой год, включая документы СССР. Документы печатаются на “правильной” бумаге самого высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригиналов. Они заверяются необходимыми печатями и штампами.
Мы готовы предложить дипломы любой профессии по доступным ценам. Цена зависит от той или иной специальности, года получения и университета. Стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступны для большинства граждан.
arusak-diploms-srednee.ru/kupit-diplom-kandidata-nauk
Хорошей учебы!
Добрый день!
Приобрести документ о получении высшего образования вы имеете возможность в нашей компании.
ast-diploms.com/kupit-diplom-moskva
Успехов в учебе!
Привет, друзья!
Наш сервис предлагает купить диплом высокого качества, неотличимый от оригинального документа без использования специального оборудования и квалифицированного специалиста.
fly-fishing.ru/forum/topic/9322/?page=1#post-46168
Успешной учебы!
Здравствуйте!
Приобрести документ о получении высшего образования можно у нас в столице.
ast-diploms.com/kupit-diplom-krasnoyarsk
Привет!
Как избежать рисков при покупке диплома колледжа или ВУЗа в России
phileo.me/blogs/new
Окажем помощь!.
Промокоды Vavada казино предоставляют игрокам эксклюзивные возможности. Эти коды помогают увеличить баланс и наслаждаться всеми преимуществами игры.
Промокоды Drip казино помогают улучшить игровой опыт. Эти коды предоставляют уникальные возможности для всех игроков, делая игру более захватывающей и интересной.
Присоединяйтесь к Vavada казино и используйте промокоды для получения бонусов. Это отличная возможность увеличить свои выигрыши и насладиться игровым процессом.
Добрый день!
Приобрести документ о получении высшего образования вы можете в нашей компании в Москве.
ast-diploms.com/kupit-diplom-sankt-peterburg
Здравствуйте!
Купить документ института вы можете в нашем сервисе.
diplomyx.com/kupit-diplom-voronezh
Успешной учебы!
Привет!
Приобрести диплом любого университета
Мы предлагаем документы техникумов, которые находятся в любом регионе РФ. Можно купить диплом от любого учебного заведения, за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригиналов. Они заверяются необходимыми печатями и штампами.
themepacific.com/support/users/lutroduyda/
Будем рады вам помочь!.
Добрый день!
Готовы предложить документы ВУЗов, которые расположены на территории всей России. Можно заказать качественный диплом от любого учебного заведения, за любой год, включая документы старого образца СССР. Дипломы и аттестаты печатаются на “правильной” бумаге высшего качества. Это дает возможности делать настоящие дипломы, которые невозможно отличить от оригинала. Они будут заверены необходимыми печатями и штампами.
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по невысоким тарифам. Цена зависит от той или иной специальности, года выпуска и образовательного учреждения. Стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступны для большинства граждан.
landik-diploms-srednee.ru/kupit-diplom-v-novosibirske В
Удачи!
Привет!
Мы предлагаем документы ВУЗов, расположенных в любом регионе России. Вы можете приобрести диплом за любой год, указав подходящую специальность и оценки за все дисциплины. Документы печатаются на бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. Документы заверяются необходимыми печатями и подписями.
cbr.by/club/user/1977/blog/329/
Привет!
Как получить диплом техникума официально и без лишних проблем
bittogether.com/index.php?action=profile;u=11883
Поможем вам всегда!.
Привет!
Всё, что нужно знать о покупке аттестата о среднем образовании
b98415ql.bget.ru/index.php?subaction=userinfo&user=yfaquvy
Поможем вам всегда!.
Привет, друзья!
Заказать диплом о высшем образовании.
glowsubs.ru/forum/messages/forum2/topic2175/message9443/?result=new#message9443
Успешной учебы!
Добрый день!
Мы предлагаем заказать диплом в высоком качестве, который не отличить от оригинального документа без использования специального оборудования и квалифицированного специалиста.
mineralgroup.ru/index.php?subaction=userinfo&user=ipygoho
Хорошей учебы!
Добрый день!
Где купить диплом специалиста?
Приобрести диплом университета.
sky-metaverse.com/read-blog/10952
Добрый день!
Мы предлагаем выгодно заказать диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти любые проверки, даже при помощи специфических приборов. Достигайте цели быстро и просто с нашими дипломами.
play.ntop.tv/index.php?subaction=userinfo&user=ilapogu
Успехов в учебе!
Здравствуйте!
Где заказать диплом специалиста?
Мы можем предложить документы институтов, расположенных в любом регионе РФ. Можно приобрести диплом за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы печатаются на бумаге самого высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригинала. Они заверяются всеми обязательными печатями и штампами.
Мы изготавливаем дипломы любой профессии по выгодным тарифам.
asxdiplomik.com/kupit-diplom-voronezh
Рады оказаться полезными!
Notice any red flags? Trust your gut. If something feels off, it’s okay to step back and reassess the situation. Your safety and comfort come first
news site
Добрый день!
Где заказать диплом по нужной специальности?
stroimsa.forum2x2.ru/t1824-topic#4732
Удачи!
Здравствуйте!
Приобрести документ о получении высшего образования вы имеете возможность у нас в столице.
asxdiplomik.com/kupit-diplom-vracha
Успешной учебы!
Привет!
Готовы предложить документы техникумов, расположенных в любом регионе РФ. Можно купить качественно сделанный диплом от любого заведения, за любой год, указав необходимую специальность и оценки за все дисциплины. Дипломы и аттестаты выпускаются на бумаге самого высшего качества. Это дает возможность делать государственные дипломы, которые невозможно отличить от оригинала. Они будут заверены всеми необходимыми печатями и подписями.
Мы предлагаем дипломы любых профессий по приятным ценам. Стоимость зависит от конкретной специальности, года получения и образовательного учреждения. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы дипломы были доступными для большинства граждан.
landik-diploms-srednee.ru/kupit-diplom-v-kazani В
Удачи!
Привет, друзья!
Как получить диплом стоматолога быстро и официально
mkpnz.ru/users/8
Будем рады вам помочь!.
Привет!
Купить диплом любого ВУЗа
landik-diploms-srednee.ru/kupit-diplom-v-omske В
Успехов в учебе!
Привет!
Заказать документ о получении высшего образования можно в нашей компании в столице.
asxdiplomik.com/kupit-diplom-ekaterinbur
Используйте промокоды Vavada казино и получайте бонусы для любимых игр. Сделайте игровой процесс еще увлекательнее с дополнительными возможностями для выигрыша.
Хотите увеличить свои шансы на выигрыш? Воспользуйтесь промокодами Drip казино и получите эксклюзивные бонусы для любимых игр.
Присоединяйтесь к Vavada казино и используйте промокоды для получения бонусов. Это отличная возможность увеличить свои выигрыши и насладиться игровым процессом.
Привет, друзья!
Мы можем предложить дипломы любых профессий по приятным тарифам.
sommos.com.co/куплю-диплом-доме/
Привет!
Где заказать диплом по актуальной специальности?
Приобрести диплом любого ВУЗа.
site2.liodiz.ru/2024/07/04/shirokiy-vybor-dokumentov-v-znamenitom-onlayn-magazine.html
Приобретение школьного аттестата с официальным упрощенным обучением в Москве
ast-diplomy.com/kupit-diplom-nizhnij-novgorod
Привет!
Купить документ о получении высшего образования можно у нас в столице.
ast-diplom.com/kupit-diplom-voronezh
Хорошей учебы!
Welcome to our Gay Blog, where we celebrate the diversity and beauty of the LGBTQ+ community. Explore articles, stories, and resources that embrace love, equality, and pride. Join us in fostering understanding, acceptance, and empowerment for all.
Здравствуйте!
Покупка диплома о среднем полном образовании: как избежать мошенничества?
girlscools.ru/vash-diplom-za-korotkoe-vremya-prosto-i-udobno
Рады помочь!.
Здравствуйте!
Приобрести диплом ВУЗа
arusak-diploms-srednee.ru/kupit-diplom-v-cheliabinske В
Успехов в учебе!
Здравствуйте!
Мы можем предложить документы техникумов, расположенных на территории всей России. Можно приобрести диплом за любой год, включая сюда документы старого образца СССР. Документы печатаются на “правильной” бумаге высшего качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. Документы заверяются всеми требуемыми печатями и подписями.
qoq.me/read-blog/143
купить диплом судоводителя ast-diploms.com .
диплом с занесением в реестр диплом с занесением в реестр .
Привет!
Сколько стоит получить диплом высшего и среднего образования легально?
ekra.kz/index.php?subaction=userinfo&user=oducafyby
Рады помочь!.
купить высшее образование с занесением в реестр купить высшее образование с занесением в реестр .
можно купить диплом высшем образовании можно купить диплом высшем образовании .
Explore exclusive Marc Jacobs discounts at the marc jacobs factory outlet online.
Добрый день!
Приобрести документ университета вы сможете в нашей компании.
ast-diplom24.ru/kupit-diplom-nizhnij-novgorod
Привет!
Купить документ института можно у нас в Москве.
diploms-x24.ru/kupit-diplom-omsk
Успехов в учебе!
Здравствуйте!
Купить диплом о высшем образовании.
http://www.easyinsurancehub.co.uk/forum/thread-5581.html
Успехов в учебе!
Привет, друзья!
Приобрести диплом университета
arusak-diploms-srednee.ru/kupit-diplom-sssr В
Успехов в учебе!
Здравствуйте!
Официальная покупка аттестата о среднем образовании в Москве и других городах
http://www.figarohair.ru/conf/viewtopic.php?f=11&t=12643&sid=84962235b947a2c9b58bbbd79fc18de8
Поможем вам всегда!.
Привет!
Мы можем предложить документы техникумов, которые находятся в любом регионе Российской Федерации. Вы сможете заказать диплом от любого заведения, за любой год, указав подходящую специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на бумаге высшего качества. Это дает возможности делать настоящие дипломы, не отличимые от оригиналов. Документы заверяются всеми требуемыми печатями и штампами.
autoplus74.com/club/user/170/blog/390/
В Vavada казино промокоды предоставляют дополнительные бонусы для всех пользователей. Улучшите свои шансы на выигрыш и наслаждайтесь игровым процессом с новыми возможностями.
В Drip казино промокоды дают возможность получить дополнительные бонусы. Используйте их для повышения шансов на выигрыш и получения удовольствия от игры.
В Vavada казино промокоды дают возможность получить дополнительные бонусы. Они помогут увеличить шансы на выигрыш и сделать игровой процесс еще увлекательнее.
Привет!
Где приобрести диплом специалиста?
Приобрести диплом университета.
kids-news.ru/diplom-pod-klyuch-kachestvenno-i-bezopasno
Hochzeitsmoderatorin
Привет, друзья!
Как получить диплом техникума с упрощенным обучением в Москве официально
allonlinesport.ru/diplomyi-lyubyih-vuzov-i-spetsialnostey/
Здравствуйте!
Где купить диплом по актуальной специальности?
Мы можем предложить документы институтов, расположенных на территории всей РФ. Вы сможете заказать качественный диплом за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы печатаются на бумаге самого высшего качества. Это позволяет делать настоящие дипломы, которые не отличить от оригиналов. Они будут заверены всеми требуемыми печатями и подписями.
Мы изготавливаем дипломы любой профессии по приятным тарифам.
ast-diploms.com/kupit-diplom-sankt-peterburg
Рады оказать помощь!
Привет!
Наша компания предлагает приобрести диплом высочайшего качества, неотличимый от оригинала без участия специалиста высокой квалификации со сложным оборудованием.
shiftdelete.10tl.net/newthread.php?fid=121
Удачи!
Здравствуйте!
Заказать документ о получении высшего образования вы имеете возможность в нашей компании. Мы предлагаем документы об окончании любых университетов Российской Федерации. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, включая документы старого образца. Гарантируем, что при проверке документа работодателем, подозрений не появится.
Мы изготавливаем дипломы любой профессии по приятным тарифам. Цена будет зависеть от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для большинства граждан.
qiqaecydybo.almoheet-travel.com/kak-vozmozno-bystro-priobresti-diplom-v-internet-magazine
Окажем помощь!
Добрый день!
Наша компания предлагает выгодно и быстро приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Документ пройдет лубую проверку, даже с применением профессиональных приборов. Решайте свои задачи максимально быстро с нашей компанией.
tatarstan.iastr.ru/index.php?subaction=userinfo&user=ufufal
Хорошей учебы!
Здравствуйте!
Приобрести документ о получении высшего образования можно в нашей компании в Москве.
ast-diplomy.com/kupit-diplom-krasnoyarsk
Добрый день!
Реально ли приобрести диплом стоматолога? Основные этапы
arusak-diploms-srednee.ru/kupit-diplom-v-krasnoiarske
Добрый день!
Узнайте стоимость диплома высшего и среднего образования и процесс получения
snaprama.com/read-blog/923
Рады оказаться полезными!
Привет, друзья!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам.
africastudygate.com/купить-диплом-тогу/
Привет!
Мы изготавливаем дипломы любой профессии по доступным тарифам.
ayhankala.com/купить-дипломы-о-высшем-образовании-в/
В Vavada казино всегда можно найти что-то новое! Используйте промокоды для получения бонусов и наслаждайтесь игровым процессом с дополнительными возможностями.
Промокоды Drip казино предоставляют уникальные бонусы, которые помогут сделать игровой процесс более интересным и захватывающим.
Промокоды Vavada казино открывают возможность для получения уникальных бонусов, что делает игровой процесс более интересным и повышает шансы на выигрыш.
Привет, друзья!
Заказать документ ВУЗа вы имеете возможность в нашем сервисе.
diplomasx.com/kupit-diplom-s-registraciej
Добрый день!
Где заказать диплом специалиста?
Купить диплом любого университета.
sklad-slabov.ru/forum/user/8261/
Добрый день!
Как не попасть впросак при покупке диплома колледжа или ПТУ в России
http://www.mamatyumen.ru/index.php?name=forums&op=showtopic&id=24393&num=1#49774
Окажем помощь!.
Добрый день!
Купить документ о получении высшего образования вы имеете возможность в нашей компании.
diploms-x.com/kupit-diplom-vracha
Удачи!
купить диплом массажиста москва diploms-x.com .
Привет!
Мы можем предложить дипломы любой профессии.
http://www.lafornacella.com/купить-диплом-переквалификации/
Рады оказать помощь!.
Привет!
Где купить диплом по необходимой специальности?
http://www.dizalty.com/read-blog/67606
Привет!
Пошаговая инструкция по официальной покупке диплома о высшем образовании
g95334gq.beget.tech/2024/07/11/vash-diplom-bez-lishnih-hlopot-i-usiliy.html
Окажем помощь!
Как купить аттестат 11 класса с официальным упрощенным обучением в Москве
ast-diploms.com/kupit-diplom-omsk
Привет, друзья!
Мы можем предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Вы сможете заказать диплом от любого заведения, за любой год, указав необходимую специальность и хорошие оценки за все дисциплины. Дипломы и аттестаты выпускаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригиналов. Они заверяются всеми требуемыми печатями и подписями.
http://www.bondhuplus.com/read-blog/81646
В Vavada казино промокоды предоставляют дополнительные бонусы, улучшая шансы на выигрыш и делая игру более увлекательной.
В Drip казино промокоды открывают доступ к дополнительным бонусам, делая игровой процесс более захватывающим.
Промокоды Vavada казино открывают доступ к уникальным бонусам, что делает процесс игры более интересным и захватывающим.
Привет, друзья!
Мы готовы предложить дипломы любой профессии по выгодным тарифам.
poobshchaemsya.ru/gallery/image/kakie-magaziny-s-diplomami-na-tekuschiy-den-imeyutsya-356/
Добрый день!
Купить документ университета
diplomasx24.ru/kupit-diplom-voronezh
Привет, друзья!
Пошаговая инструкция по официальной покупке диплома о высшем образовании
arusak-diploms-srednee.ru/kupit-attestat-za-11-klass В
Добрый день!
Приобрести диплом о высшем образовании.
gamesmaker.ru/forum/common/offtopics/#replyform
Успехов в учебе!
Добрый день!
Приобрести документ университета можно в нашем сервисе.
ast-diplomas.com/kupit-diplom-krasnoyarsk
Успехов в учебе!
Добрый день!
Купить диплом о среднем образовании в Москве и любом другом городе
datasphere.ru/club/user/15/blog
Рады оказать помощь!
Здравствуйте!
Купить документ ВУЗа вы сможете в нашей компании в Москве.
asxdiplomik.com/kupit-diplom-tehnikuma-kolledzha
Здравствуйте!
Где купить диплом специалиста?
Купить диплом о высшем образовании.
klinlife.ru/forums/index.php?/topic/41391-legkii-sposob-poluchit-diplom-bez-akzamenov/
Привет!
Мы предлагаем документы ВУЗов, расположенных в любом регионе России. Вы имеете возможность купить качественно сделанный диплом за любой год, включая сюда документы старого образца СССР. Документы делаются на “правильной” бумаге высшего качества. Это дает возможность делать настоящие дипломы, которые не отличить от оригиналов. Документы заверяются всеми обязательными печатями и подписями.
hymaxonelae.theglensecret.com/kak-mozno-budet-nedorogo-kupit-attestat-v-internet-magazine
Здравствуйте!
Мы изготавливаем дипломы любых профессий по выгодным тарифам.
toplentanews.ru/oformlenie-diploma-onlayn-za-neskolko-shagov
Привет, друзья!
Заказать документ института вы имеете возможность в нашем сервисе.
ast-diplom24.ru/kupit-diplom-nizhnij-novgorod
Успехов в учебе!
Здравствуйте!
Сколько стоит диплом высшего и среднего образования и как это происходит?
profit.hutt.live/viewtopic.php?id=1979#p4278
Поможем вам всегда!
Здравствуйте!
Официальная покупка диплома вуза с сокращенной программой обучения в Москве
goebecom.jofo.me/2268562.html
Рады оказаться полезными!.
Привет!
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам.
gamemotors.ru/diplomyi-s-polnyim-paketom-dokumentov-2
Привет, друзья!
Мы можем предложить документы ВУЗов, которые расположены на территории всей РФ. Можно заказать качественный диплом за любой год, указав необходимую специальность и оценки за все дисциплины. Документы делаются на бумаге самого высокого качества. Это позволяет делать государственные дипломы, которые невозможно отличить от оригинала. Документы заверяются необходимыми печатями и подписями.
menaharia.com/read-blog/2713
Looking for a quick financial boost? Apply now for a advance cash loan online and secure the funds you need with ease. Whether it’s for unexpected expenses, home improvements, or a special purchase, our fast approval process ensures you get the money swiftly. Don’t wait – take the first step towards financial freedom today!
Привет, друзья!
Заказать документ института можно у нас в Москве.
diplomyx.com/kupit-diplom-kandidata-nauk
Привет!
Купить документ университета можно в нашей компании в Москве.
ast-diplomy24.ru/otzyvy
Успешной учебы!
Привет, друзья!
Заказать диплом ВУЗа.
x91392sl.beget.tech/2024/05/17/kupit-diplom-vash-shans-stat-liderom-v-svoey-oblasti.html
Хорошей учебы!
Добрый день!
Где купить диплом специалиста?
kryza.network/read-blog/11426191
Быстрая схема покупки диплома старого образца: что важно знать?
ast-diplom24.ru/otzyvy
Чай від застуди – це корисний напій, що виготовляють з різних лікарських рослин. Настій лікувальний для організму, оскільки має багато вітамінів.
Трав’яні чаї можна купити в магазині AD-UA в Україні. Магазин пропонує різноманітні види трав’яного чаю на будь-який смак.
Переходь на сайт – https://ad-ua.com/chaj/trav-yanyj-chaj/
Добрый день!
Приобрести документ института вы имеете возможность в нашем сервисе.
asxdiplomik24.ru/kupit-diplom-moskva
Хорошей учебы!
Привет, друзья!
Диплом вуза купить официально с упрощенным обучением в Москве
gamesfortop.ru/vash-diplom-garantiya-kachestva-i-podlinnosti
Добрый день!
Заказать документ о получении высшего образования
diplomasx24.ru/kupit-diplom-magistra
Привет!
Всё, что нужно знать о покупке аттестата о среднем образовании
arusak-diploms-srednee.ru В
Привет, друзья!
Заказать документ ВУЗа можно у нас в столице.
asxdiplomik.com/kupit-diplom-krasnodar
Здравствуйте!
Мы предлагаем дипломы любой профессии по приятным ценам.
karhu.blueaddlution.com/2024/06/30/купить-диплом-в-серове/
Привет, друзья!
Официальная покупка школьного аттестата с упрощенным обучением в Москве
mariland.org/index.php?subaction=userinfo&user=aduzu
Рады оказать помощь!.
Здравствуйте!
Купить документ университета вы имеете возможность у нас в столице.
diploms-x24.ru/kupit-diplom-voronezh
Успехов в учебе!
Привет!
Рекомендации по безопасной покупке диплома о высшем образовании
remotebillpay.com/купить-диплом-о-высшем-образовании-ре/
Поможем вам всегда!.
Discover the thrill of winning big at our premier online casino usa real money no deposit, where every spin of the wheel promises excitement and opportunity. Immerse yourself in a world of luxury and glamour, where the lights are bright, and the stakes are high. Our casino offers an unparalleled gaming experience with a vast selection of classic and modern games tailored for all levels of players. Join us and feel the adrenaline rush as you take your chance at fortune in an atmosphere of elegance and sophistication.
Здравствуйте!
Купить диплом любого университета.
ccde-cambodia.com/cb-profile/pluginclass/cbblogs.html?action=blogs&func=show&id=118
buy lasuna cheap – buy himcolin pill buy generic himcolin for sale
cp loli irish colleen
==> biturl.top/qeAJJf rlys.nl/6epap3 <==
Добрый день!
Заказать документ о получении высшего образования можно у нас в столице.
ast-diplomas.com/kupit-diplom-tehnikuma-kolledzha
Привет, друзья!
Приобретение диплома ПТУ с сокращенной программой обучения в Москве
vintfint.com/blogs/21171/ упите-?ипломы-Ѕыстро-и-Ќадежно
Become our affiliate
https://affiliates.whalehunter.cash/track/Kirill.18AffRef.18AffRef.MAIN.0.0.0.0.0.0.0.0
Привет!
Заказать документ о получении высшего образования вы имеете возможность в нашей компании.
ast-diplomas.com/kupit-diplom-magistra
Хорошей учебы!
Привет!
Мы изготавливаем дипломы любых профессий по выгодным тарифам.
http://www.polon-roof.ro/uncategorized/купить-копии-дипломов/
Привет!
Заказать документ университета можно в нашей компании.
ast-diplomy24.ru/kupit-diplom-omsk
Успехов в учебе!