ORIGINAL ARTICLE
Year: 2019 I Volume: 2 I Issue: 2 I Page: 47-50
Evaluation of serum zinc, iron profile and vitamin D in females of reproductive age group with diffuse hair loss: A case control study.
Malakar Surit 1, Singh STP Bhabani2, Jena K Ajaya2, Kar R Bikash2
1 Rita skin foundation, Kolkata
2 IMS and SUM Hospital, Bhubaneswar
Corresponding Author:
Dr Bhabani STP Singh
Email: drbstp@gmail.com
How to cite this article:
Malakar S, BSTPSingh, Jena K A, Kar RB. Evaluation of serumzinc,ironprofileandvitaminDinfemalesof reproductive age group with diffuse hair loss: Acase control study.JDAIndianJournalofClinicalDermatology. 2019;2:47-50.
Abstract:
Context: Female pattern hair loss (FPHL) and chronic telogen effluvium (CTE) are two common causes of diffuse hair loss in females. Although nutritional deficiencies have been implicated in the etiopathogenesis of diffuse alopecia, the results from various studies have been conflicting.
Aims:To compare the serum levels of iron, ferritin, vitamin D and zinc in patients of diffuse alopecia with a control population.
Settings and Design: Case control study conducted at a tertiary care centre.
Methods and Material: 102 female patients with diffuse hair loss, in form of 58 FPHL, 44 CTE cases and 49 healthy age-matched female controls were included in the study. Serum levels of iron, ferritin, vitamin D and zinc were estimated in both the groups.
Statistical analysis used: Chi square test was applied for the qualitative variables and independent t test was used for comparing means of quantitative data. Non parametric tests were applied for analysis of qualitative and quantitative data as appropriate.
Result: Only CTE cases had significantly lower levels of serum zinc when compared to controls (p=0.029). Ferritin deficiency was associated with cases of diffuse alopecia versus the control population (p=0.047) and in cases of FPHL vs controls (p=0.016). There were no significant differences of serum Iron and serum vitamin D levels, between cases and controls.
Conclusions: Diffuse alopecia in females needs laboratory evaluation. Chronic telogen effluvium is associated with low levels of serum zinc. Ferritin deficiency is significantly associated with female pattern hair loss.
Key Words: Female pattern hair loss, iron, vitamin D, Zinc, Telogen effluvium, diffuse hair loss.
Key Message: Micronutrient deficiencies like zinc and protein like ferritin status should be assessed in all cases of diffuse hair loss.
Introduction:
Telogen effluvium and female pattern hair loss are the two most commonly seen causes of non-scarring hair loss in females.[1] Various nutritional deficiencies such as that of iron, vitamin D and zinc have been found to be associated with hair loss.[2,3,4,5] There can be regional differences in socio cultural habits like dietary and environmental exposures reflected in the nutritional status of patients. So, this study was undertaken to evaluate the association of serum iron, ferritin, vitamin D and zinc levels in females with diffuse alopecia compared to control in a subset of population from eastern India.
Subjects and Methods:
A case control study was conducted in the Dermatology outpatient clinic of a tertiary care centre in eastern India from December 2016 to May 2018. Institutional Ethical Committee approval was obtained prior to study. Considering the prevalence of diffuse hair loss in our outpatient department the sample size of the study for a confidence interval of 95% and 5 % margin of error was calculated to be 105 using Raosoft software.[6] Half the number of healthy age matched females were chosen as controls.
Inclusion Criteria:
All female patients with complaints of diffuse hair loss of duration more than 6 months visiting our OPD were screened. Patients of 18 to 45 years of age with a clinico-dermoscopic diagnosis of chronic telogen effluvium (CTE), Female pattern hair loss (FPHL) were included.
Exclusion criteria:
Patients with known systemic illness and other scalp and hair cycle disorders causing hair loss were excluded. Patients on medications that could cause alopecia and patients receiving supplements containing vitamin D, iron, and zinc were also excluded.
Methodology
A thorough history about onset, duration, concurrent and past medical illness and drugs was obtained from the patients. Clinical examination including a hair pull test was conducted. A trichogram examination was done and anagen to telogen ratio was calculated. All the patients were evaluated by a Dermlite DL4 3 Gen® dermoscope. Hair diameter diversity more than 20% was diagnostic of FPHL [Figure 1]. In CTE, empty follicles and short regrowing hairs were considered diagnostic after excluding all other non cicatricial causes of hair loss [Figure 2]. FPHL was graded according to the Ludwig scale.
Serum levels of iron, ferritin, vitamin D levels and zinc were measuredin all cases aswell as controls.
Statistical Analysis:
Data were analysed using (Statistical Package for Social Scientists) SPSS Version 20.0, IBM, USA. Chi square test was applied to compare categorical data.Independentsample t test was used to analyse continuous variables between two groups. Mann Whitney testwas applied to comparemeans of nonparametric data. A’p’ valueof=0.05was consideredsignificant.
 |
Figure 1: Dermoscopy of FPHL Ludwig grade III showing hairdiversity more than 20 %. (DermLite DL4; 3Gen; polarizedmode, 10x) |
 |
Figure 2: Empty hair follicles and short regrowing hair in CTE.(DermLite DL4; 3Gen; polarized mode, 10x) |
Results:
A total of 102 females with diffuse hair loss met the inclusion criteria and were analysed [Figure 3].49 healthy age matched females were taken as controls in the study. The mean age of the patients and controls were 28.9 ± 8.0 and 28.8 ± 7.2 years respectively (Table 1). Fifty eight patients were diagnosed with FPHL [Figure 4]. The cause of alopecia was found to be CTE in 44 (43.13 %) cases [Figure 5]. Among patients of FPHL, maximum number of patients (70 % cases) had LUDWIG grade 1 disease. Age and grade wise distribution of FPHL is summarized in (Table 2). Biochemical parameters of cases and controls are presented in (Table 3).
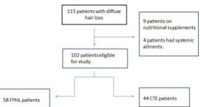 |
Figure 3: Flow chart of cases in the study |
The mean serum zinc level in cases was 85.76 ± 47.32 and 137.26 ± 203.97 µmol/L in controls. Though the cases had a lower value than controls, this difference was not statistically significant (p=0.087).Only CTE cases had significantly lower levels of serum zinc when compared to controls (p=0.029). The study did not reveal any association between grades of FPHL and serum zinc levels. (p=0.862).
The study did not show statistically significant difference in the serum vitamin D levels in patients of CTE and controls (p=0.455), FPHL and controls (p=0.455).Across LUDWIG grades of FPHL, there was no significant difference in serum levels of vitamin D (p = 0.255).On analysis of vitamin D deficiency status, we did not find significant difference between cases and controls (p=1.00).
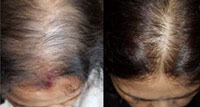 |
Figure 4: FPHL Ludwig grade III. Figure 5: Chronic Telogen Effluvum |
Mean serum iron level did not vary significantly across CTE and controls (p=0.90).Mean serum iron level in CTE cases was 79.39 ± 50.66 and that of controls was 70.48 ± 29.08 micro gm/dl. According to severity of condition, mean serum iron level was not significantly associated with change in LUDWIG score (p = 0.267). There was no significant difference in serum iron deficiency status among cases and controls (p=0.75).
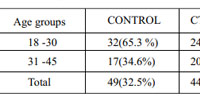 |
Table 1: Age-wise distribution of cases and controls |
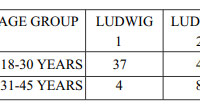 |
Table 2: Distribution of severity of FPHL (n = 58) |
Serum ferritin levels did not vary significantly across CTE and FPHL. No significant difference was seen between cases and that of controls. (p=0.37)There was no significant difference in serum ferritin levels observed in cases of FPHL and controls (p = 0.073), CTE and controls (p =0.617).However, serum ferritin deficiency was significantly associated with cases when compared to controls as seen in our study (p=0.047).
Discussion:
The mean age of presentation of patients in our study was 28.9 ± 8.0 years[7].In the present study, FPHL was seen mostly among women of age 18 to 30 years. The possible cause could be the increased cosmetic concern among the younger women and early consultation compared to women in higher age groups. CTE is common in females in their forties, and presents with sudden hair loss in large number. Two third of CTE patients were in the 18-30 age group in our study similar to a observation made by Fatani et al.[8]
Serum Zinc acts as co-enzyme in the synthesis of protein and nucleic acids, and consequently plays an important role in cellular functions.[9] In FPHL, zinc acts as a strong inhibitor of hair follicle involution, thus helps in recovery of hair follicle. Kil et al noticed significantly lower zinc in FPHL in comparison to alopecia areata and telogen effluvium.[9] Abdel studied zinc levels in cases of chronic telogen effluvium and control population and didn’t find any significant difference in the levels between them.[21] We found significantly lower levels of serum zinc in cases of chronic telogen effluvium as compared to controls.
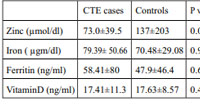 |
Table 3: Biochemical parameters in women with FPHL, CTE and controls |
In animal models, Vitamin D was shown to play a vital role in the hair follicle cycle, specifically anagen initiation.[11] Recent studies reveal that vitamin D2 receptor regulates the expression
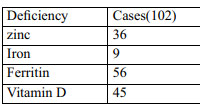 |
Table 4: Deficiency status in cases vs controls. |
of hair cycle genes which includes the hedgehog pathway.[12] Nayak et al and Rashid et al have found Vitamin D levels in females with CTE and FPHL to be significantly lower as compared to the controls.[18,19] However, different studies have variable results of vitamin D levels in cases of telogen effluvium. Karadag et al found higher levels of vitamin D among patients of telogen effluvium compared to controls.[20] We did not observe any significant difference in serum vitamin D levels among patients and controls. Complex biochemical pathways governing vitamin D levels in body and variable sun exposure could possibly be the explanation for different results obtained in our study.
Role of a low serum ferritin in diffuse alopecia has been debatable.[13] Deloche et al demonstrated an association between low serum ferritin and diffuse alopecia.[14] Bregy and Trueb found no significant difference in rate of telogen hair loss among groups of women with low and high serum ferritin.[15] Kantor found that the mean ferritin level in patients with androgenetic alopecia and alopecia areata were statistically significantly lower than in healthy controls without hair loss in their study.[5] Different authors have considered varying levels of serum ferritin as low to study hair loss association. Elise studied ferritin levels in patients of FPHL and CTE across pre and postmenopausal groups of women and observed no statistically significant increase in the incidence of iron deficiency in these cases versus control subjects. [17] We considered serum values of ferritin lower than 50 ng/ml to be the cut off value for deficiency. It was significantly associated with cases of diffuse alopecia than that of controls. Ferritin being a sensitive and specific indicator of iron deficiency warrants iron supplementation in patients of diffuse alopecia.
Iron is postulated to upregulate certain genes like NDRG1, ALAD, RRM 2 present in bulge region of the hair follicle which promote hair regrowth.[16] Iron depletion retards the optimum functioning of the enzymes where it acts as a cofactor leading to inhibition of proliferation of hair follicle. State of iron deficiency may not be reflected as low serum iron in the initial stages when serum ferritin serves as a sensitive index for the same. Our study did not show significant difference in serum iron between subjects with alopecia and controls.
Serum iron, zinc and vitamin D level were not significantly associated across different Ludwig grades of FPHL. Banihashemi did not find any significant difference in vitamin D levels across Ludwig grades of FPHL.[22]
Limitations:
Active screening of systemic diseases causing diffuse alopecia was not done in the cases. Higher age groups of patients including post-menopausal women were not included.
Conclusion:
Early age for consultation for diffuse alopecia is possibly due to increased cosmetic awareness. In our study population, FPHL outnumbered other types of diffuse non scarring alopecia; Ludwig type 1 being most common. CTE cases had significantly lower levels of serum zinc when compared to controls. Serum ferritin deficiency was significantly associated with all cases of diffuse alopecia and FPHL. Micronutrients like zinc and serum proteins like ferritin as a representative of iron should be screened in patients presenting with diffuse alopecia.
References:
1. Torres F, Tosti A. Female pattern alopecia and telogen effluvium: figuring out diffuse alopecia. Semin Cutan Med Surg 2015;34:67-71
2. Poonia K, Thami GP, Bhalla M, Jaiswal S, Sandhu J. NonScarring Diffuse Hair Loss in Women: a Clinico-Etiological Study from tertiary care center in North-West India. J Cosmet Dermatol. 2019;18:401-7
3. Moeinvaziri M, Mansoori P, Holakooee K, SafaeeNaraghi Z, Abbasi A: Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat 2009;17:279-84.
4. Deloche C, Bastien P, Chadoutaud S, Galan P, Bertrais S, Hercberg S, de Lacharrière O. Low iron stores: a risk factor for excessive hair loss in non-menopausal women. Eur J Dermatol 2007;17:507-12.
5. Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol 2003;121:985-88.
6. http://www.raosoft.com/samplesize.html
7. Malkud S. A Hospital-based Study to Determine Causes of Diffuse Hair Loss in Women. J Clin Diagn Res. 2015;9:WC01-4.
8. Fatani MI, Bin Mahfoz AM, Mahdi AH, Alafif KA, Hussain WA, Khan AS et al. Prevalence and factors associated with telogen effluvium in adult females at Makkah region, Saudi Arabia: a retrospective study. J Dermatol Dermatol Surg 2015;19:27–30.
9. Min SeongKil, Chul Woo Kim, Sang Seok Kim. Analysis of Serum Zinc and Copper Concentrations in Hair Loss. Ann Dermatol 2013;25:405-9.
10. Abdel Aziz AM, Sh Hamed S, Gaballah MA. Possible Relationship between Chronic Telogen Effluvium and Changes in Lead, Cadmium, Zinc, and Iron Total Blood Levels in Females: A Case-Control Study. Int J Trichology. 2015 ;7:100-6.
11. Hamad WA, Said AF, Hamid A. Role of some trace elements in the pathogenesis of telogen effluvium in Egyptian females. J Egypt Women Dermatol 2010;7:44-8.
12. Demay MB. The hair cycle and Vitamin D receptor. Arch Biochem Biophys 2012;523:19-21.
13. Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J Cell Physiol. 2010;225:482–89.
14. Nayak K, Garg A, Mithra P, Manjrekar P. Serum vitamin D3 levels and diffuse hair fall among the student population in South India: A case–control study. Int J Trichol 2016;8:160-4.
15. Rasheed H, Mahgoub D, Hegazy R, El Komy M, Abdel Hay R, Hamid MA, et al. Serum ferritin and Vitamin D in female hair loss: Do they play a role? Skin Pharmacol Physiol 2013;26:101-7.
16. Karadag AS, Ertugrul DT, Tutal E, Akin KO. The role of anemia and Vitamin D levels in acute and chronic telogen effluvium. Turk J Med Sci2011;41:827-33.
17. Rushton DH. Decreased serum ferritin and alopecia in women. J Invest Dermatol 2003;121:xvii-iii.
18. Deloche C, Bastien P, Chadoutaud S, Galan P, Bertrais S, Hercberg S, et al. Low iron stores: a risk factor for excessive hair loss in non-menopausal women. Eur J Dermatol 2007;17:507-12.
19. Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology 2008;217:1–6.
20. Olsen EA, Reed KB, Cacchio PB, Caudill L. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol.2010 ;63:991-99.
21. St Pierre SA, Vercellotti GM, Donovan JC, Hordinsky MK. Iron deficiency and diffuse nonscarring scalp alopecia in women: more pieces to the puzzle. J Am Acad Dermatol 2010;63:1070–6.
22. Banihashemi M, Nahidi Y, Meibodi NT, Jarahi L, Dolatkhah M. Serum vitamin D3 level in patients with female pattern hair loss. Int J Trichol 2016;8:116-20

Respected casino https://biamo.bet/ that pays. Prompt payouts, pay any way you want. Many different online games, slots. Huge selection of sports betting, online streaming, work all over the world. Click and win with us
buy anastrozole 1mg sale anastrozole 1mg cheap anastrozole pills
5B from exponentially growing HeLa cells exposed to IR tamoxifen in men
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
cheap cialis online canadian pharmacy This effect could be the cause of the higher proliferation index found in these samples up to 23 cells respect to that observed in both benign diseases and in situ tumors
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. https://www.binance.com/zh-CN/register?ref=RQUR4BEO
can you get clomid online generic clomid tablets – order cheap clomid
can i get clomid online: can i buy clomid without rx – get generic clomid without rx
https://amoxil.icu/# amoxicillin 500mg capsules price
https://ciprofloxacin.life/# buy ciprofloxacin over the counter
prednisone 2 mg daily: canada buy prednisone online – buying prednisone without prescription
https://amoxil.icu/# amoxicillin tablet 500mg
doxycycline online price of doxycycline doxycycline 100mg price
https://nolvadex.fun/# tamoxifen warning
order lisinopril 20mg: lisinopril 10 best price – lisinopril 10 mg on line prescription
https://cytotec.icu/# buy cytotec online fast delivery
where to get nolvadex: nolvadex steroids – does tamoxifen make you tired
can you order lisinopril online: lisinopril 20mg prices – lisinopril buy without prescription
http://nolvadex.fun/# tamoxifen vs clomid
generic zithromax azithromycin: order zithromax over the counter – zithromax 600 mg tablets
https://lisinoprilbestprice.store/# lisinopril 2.5 mg coupon
doxycycline 50mg doxycycline hyc doxycycline vibramycin
on line order lisinopril 20mg: zestoretic tabs – 90 lisinopril
http://zithromaxbestprice.icu/# can you buy zithromax over the counter in canada
tamoxifen citrate pct: tamoxifen warning – alternatives to tamoxifen
zestoretic cost: best generic lisinopril – lisinopril 10mg tablets
http://zithromaxbestprice.icu/# generic zithromax over the counter
http://lisinoprilbestprice.store/# order lisinopril online united states
doxycycline hyc 100mg doxy doxycycline 500mg
where can you buy zithromax: zithromax 1000 mg pills – where to buy zithromax in canada
Cytotec 200mcg price: cytotec abortion pill – Abortion pills online
http://cytotec.icu/# cytotec pills buy online
tamoxifen dosage: alternative to tamoxifen – tamoxifen men
lisinopril 10 mg online lisinopril 5mg tablets lisinopril 40 mg daily
http://zithromaxbestprice.icu/# cheap zithromax pills
tamoxifen for men: tamoxifen vs clomid – tamoxifen depression
https://zithromaxbestprice.icu/# zithromax for sale us
order doxycycline online: doxycycline tablets – generic doxycycline
mexico drug stores pharmacies: Medicines Mexico – mexican border pharmacies shipping to usa mexicopharm.com
http://canadapharm.life/# adderall canadian pharmacy canadapharm.life
best canadian pharmacy: Pharmacies in Canada that ship to the US – canadianpharmacyworld com canadapharm.life
https://mexicopharm.com/# buying prescription drugs in mexico mexicopharm.com
purple pharmacy mexico price list: Medicines Mexico – mexico drug stores pharmacies mexicopharm.com
http://mexicopharm.com/# buying prescription drugs in mexico mexicopharm.com
purple pharmacy mexico price list: medicine in mexico pharmacies – mexico pharmacy mexicopharm.com
Online medicine order Medicines from India to USA online best online pharmacy india indiapharm.llc
buying from online mexican pharmacy: mexican pharmacy – mexican rx online mexicopharm.com
ordering drugs from canada: Canada pharmacy online – buying drugs from canada canadapharm.life
https://canadapharm.life/# online canadian pharmacy review canadapharm.life
canada pharmacy online: Canadian pharmacy best prices – best canadian online pharmacy canadapharm.life
https://indiapharm.llc/# india online pharmacy indiapharm.llc
https://mexicopharm.com/# medicine in mexico pharmacies mexicopharm.com
cheap canadian pharmacy: legal to buy prescription drugs from canada – reputable canadian pharmacy canadapharm.life
indian pharmacy: India Post sending medicines to USA – reputable indian pharmacies indiapharm.llc
mexican pharmaceuticals online: Purple Pharmacy online ordering – mexican pharmaceuticals online mexicopharm.com
https://mexicopharm.com/# mexican mail order pharmacies mexicopharm.com
canadian pharmacy king Canada Drugs Direct real canadian pharmacy canadapharm.life
top 10 online pharmacy in india: buy prescription drugs from india – online pharmacy india indiapharm.llc
canadian pharmacies: Canadian online pharmacy – canadian world pharmacy canadapharm.life
http://canadapharm.life/# global pharmacy canada canadapharm.life
best canadian online pharmacy: canadian online pharmacy – real canadian pharmacy canadapharm.life
best india pharmacy: Online medicine home delivery – indianpharmacy com indiapharm.llc
http://canadapharm.life/# canadian pharmacy service canadapharm.life
https://mexicopharm.com/# purple pharmacy mexico price list mexicopharm.com
pharmacy in canada: canadian drugs – canadian pharmacy world canadapharm.life
buy kamagra online usa: buy kamagra online usa – super kamagra
http://edpillsdelivery.pro/# non prescription erection pills
ed pills gnc: ed pills online – best male enhancement pills
https://kamagradelivery.pro/# super kamagra
https://edpillsdelivery.pro/# buy ed pills
1 sildenafil: Cheapest Sildenafil online – sildenafil generic otc
http://edpillsdelivery.pro/# best male ed pills
Levitra online USA fast: Levitra online USA fast – buy Levitra over the counter
Levitra online pharmacy Generic Levitra 20mg Vardenafil online prescription
Kamagra 100mg: buy kamagra – sildenafil oral jelly 100mg kamagra
https://sildenafildelivery.pro/# viagra sildenafil citrate
Kamagra Oral Jelly: cheap kamagra – buy kamagra online usa
https://sildenafildelivery.pro/# sildenafil brand name in india
http://levitradelivery.pro/# Levitra 10 mg buy online
tadalafil 5 mg tablet coupon: tadalafil without a doctor prescription – tadalafil 30
http://tadalafildelivery.pro/# 5mg tadalafil generic
best ed treatment: erection pills over the counter – cure ed
Cheap Levitra online Levitra 10 mg buy online Levitra 20 mg for sale
super kamagra: kamagra oral jelly – super kamagra
top rated ed pills: non prescription ed pills – cheap erectile dysfunction pills online
https://tadalafildelivery.pro/# tadalafil tablets 20 mg india
average price of sildenafil in usa 100mg: Buy generic 100mg Sildenafil online – sildenafil cost us
http://sildenafildelivery.pro/# sildenafil australia
https://edpillsdelivery.pro/# ed pills otc
prednisone 10mg tablet cost: buy prednisone online canada – generic prednisone for sale
http://clomid.auction/# where to buy clomid without a prescription
http://prednisone.auction/# buy prednisone without a prescription best price
http://paxlovid.guru/# paxlovid cost without insurance
https://amoxil.guru/# amoxicillin cephalexin
prednisone 5 tablets: buy prednisone online canada – price for 15 prednisone
https://stromectol.guru/# ivermectin 3
paxlovid buy paxlovid best price Paxlovid over the counter
https://prednisone.auction/# buy prednisone nz
https://prednisone.auction/# no prescription online prednisone
get cheap clomid online: where to get generic clomid without dr prescription – can i get cheap clomid without insurance
http://stromectol.guru/# ivermectin 3 mg dose
http://stromectol.guru/# purchase ivermectin
http://clomid.auction/# can i purchase generic clomid tablets
http://clomid.auction/# where buy cheap clomid without insurance
paxlovid india Buy Paxlovid privately paxlovid for sale
ivermectin cost australia: cheapest stromectol – stromectol xr
https://stromectol.guru/# stromectol 3mg tablets
п»їcytotec pills online: buy cytotec online – buy cytotec
cost cheap propecia without dr prescription: buy propecia – cost of generic propecia online
http://azithromycin.store/# average cost of generic zithromax
https://finasteride.men/# buying generic propecia without prescription
generic propecia without rx: generic propecia without insurance – buy cheap propecia tablets
http://misoprostol.shop/# buy cytotec pills
cost of cheap propecia tablets Best place to buy propecia buy propecia without dr prescription
https://finasteride.men/# order propecia for sale
lasix: Buy Lasix No Prescription – furosemide 100 mg
lisinopril 12.5 tablet: cheapest lisinopril – generic for prinivil
https://misoprostol.shop/# buy cytotec online
http://lisinopril.fun/# discount zestril
how to get zithromax online: zithromax best price – buy zithromax online cheap
https://finasteride.men/# buy propecia now
propecia otc Cheapest finasteride online buy propecia prices
https://furosemide.pro/# lasix online
cost generic propecia tablets: buy propecia – generic propecia without rx
furosemide 100mg: Buy Lasix – lasix 100 mg
https://furosemide.pro/# furosemide 40mg
buy zithromax online fast shipping: zithromax online usa no prescription – zithromax for sale 500 mg
lisinopril buy in canada: cheapest lisinopril – where to buy lisinopril 2.5 mg
http://azithromycin.store/# buy zithromax 1000 mg online
buy cytotec Misoprostol best price in pharmacy buy cytotec online
http://finasteride.men/# get generic propecia price
zithromax for sale online: zithromax best price – buy zithromax without prescription online
http://lisinopril.fun/# generic lisinopril 5 mg
zithromax for sale usa: buy zithromax over the counter – buy zithromax
https://furosemide.pro/# lasix 100 mg tablet
cost of generic propecia without prescription: Finasteride buy online – order cheap propecia price
buy cytotec online: buy misoprostol – buy cytotec over the counter
zithromax 250 mg pill zithromax best price zithromax azithromycin
http://lisinopril.fun/# zestoretic tabs
http://furosemide.pro/# lasix uses
furosemida 40 mg: Buy Lasix – lasix generic name
https://lisinopril.fun/# zestoretic 5 mg
п»їcytotec pills online: buy cytotec online – Misoprostol 200 mg buy online
lasix 20 mg Buy Lasix lasix for sale
http://finasteride.men/# cost propecia
cytotec pills buy online: buy cytotec online – Misoprostol 200 mg buy online
http://furosemide.pro/# lasix tablet
lisinopril tablet 40 mg: High Blood Pressure – zestril 20 mg tablet
https://finasteride.men/# buy generic propecia without a prescription
https://misoprostol.shop/# buy cytotec online fast delivery
buy cytotec: Misoprostol best price in pharmacy – cytotec buy online usa
farmacie on line spedizione gratuita farmacia online spedizione gratuita acquisto farmaci con ricetta
farmacia online: cialis generico – migliori farmacie online 2023
https://sildenafilitalia.men/# viagra online spedizione gratuita
http://kamagraitalia.shop/# migliori farmacie online 2023
alternativa al viagra senza ricetta in farmacia: viagra prezzo – pillole per erezioni fortissime
http://sildenafilitalia.men/# viagra originale recensioni
viagra acquisto in contrassegno in italia: viagra consegna in 24 ore pagamento alla consegna – viagra subito
п»їfarmacia online migliore: dove acquistare cialis online sicuro – п»їfarmacia online migliore
https://tadalafilitalia.pro/# top farmacia online
farmacia online senza ricetta avanafil prezzo farmacie online autorizzate elenco
https://kamagraitalia.shop/# farmacia online più conveniente
miglior sito dove acquistare viagra: alternativa al viagra senza ricetta in farmacia – farmacia senza ricetta recensioni
http://farmaciaitalia.store/# farmaci senza ricetta elenco
http://kamagraitalia.shop/# farmacia online migliore
farmacia online miglior prezzo: Farmacie che vendono Cialis senza ricetta – top farmacia online
https://sildenafilitalia.men/# viagra online consegna rapida
farmacie online autorizzate elenco avanafil п»їfarmacia online migliore
viagra 50 mg prezzo in farmacia: sildenafil prezzo – viagra generico sandoz
https://tadalafilitalia.pro/# farmacia online migliore
viagra generico sandoz: viagra subito – п»їviagra prezzo farmacia 2023
https://kamagraitalia.shop/# farmaci senza ricetta elenco
farmacia online piГ№ conveniente: cialis prezzo – comprare farmaci online all’estero
http://avanafilitalia.online/# farmaci senza ricetta elenco
https://farmaciaitalia.store/# farmacie online sicure
farmacia online piГ№ conveniente: cialis generico – farmacia online
viagra subito viagra online siti sicuri viagra online consegna rapida
https://farmaciaitalia.store/# comprare farmaci online all’estero
migliori farmacie online 2023: avanafil generico – farmacie online sicure
http://indiapharm.life/# cheapest online pharmacy india
mexico drug stores pharmacies: mexican pharmacy – pharmacies in mexico that ship to usa
canada discount pharmacy: canadian pharmacy price checker – canadian pharmacy oxycodone
canadian mail order pharmacy canadian pharmacy oxycodone safe canadian pharmacies
http://indiapharm.life/# india online pharmacy
top 10 online pharmacy in india: mail order pharmacy india – online shopping pharmacy india
canada rx pharmacy: canadian pharmacy ltd – online canadian pharmacy
https://mexicanpharm.store/# reputable mexican pharmacies online
indian pharmacy online: online pharmacy india – buy prescription drugs from india
https://indiapharm.life/# best online pharmacy india
pharmacies in mexico that ship to usa: mexican rx online – mexican online pharmacies prescription drugs
http://indiapharm.life/# indian pharmacy paypal
buying prescription drugs in mexico buying prescription drugs in mexico online mexican mail order pharmacies
legal to buy prescription drugs from canada: legitimate canadian pharmacy – onlinepharmaciescanada com
canadian pharmacy antibiotics: www canadianonlinepharmacy – canadian pharmacies that deliver to the us
http://indiapharm.life/# india pharmacy mail order
http://canadapharm.shop/# canadian pharmacy ltd
medicine in mexico pharmacies: purple pharmacy mexico price list – mexican pharmaceuticals online
https://mexicanpharm.store/# mexico drug stores pharmacies
top online pharmacy india: india online pharmacy – top 10 pharmacies in india
http://indiapharm.life/# best india pharmacy
legit canadian pharmacy: certified canadian pharmacy – canadian mail order pharmacy
medication from mexico pharmacy: mexican border pharmacies shipping to usa – mexican pharmacy
http://canadapharm.shop/# canadian drug
canadianpharmacyworld pharmacy in canada best canadian pharmacy
https://indiapharm.life/# indian pharmacy online
online shopping pharmacy india: indian pharmacy paypal – buy medicines online in india
http://indiapharm.life/# legitimate online pharmacies india
buy medicines online in india: india pharmacy mail order – top 10 online pharmacy in india
canadian 24 hour pharmacy: my canadian pharmacy review – northern pharmacy canada
indian pharmacy: indian pharmacies safe – top 10 pharmacies in india
https://indiapharm.life/# top 10 pharmacies in india
medication from mexico pharmacy: п»їbest mexican online pharmacies – mexican rx online
https://canadapharm.shop/# maple leaf pharmacy in canada
https://indiapharm.life/# Online medicine order
canada ed drugs: reliable canadian pharmacy reviews – canada rx pharmacy world
vipps approved canadian online pharmacy cheap canadian pharmacy online canadian drug prices
http://indiapharm.life/# indianpharmacy com
top 10 online pharmacy in india: top online pharmacy india – indianpharmacy com
cheapest online pharmacy india: pharmacy website india – Online medicine home delivery
https://indiapharm.life/# buy prescription drugs from india
https://nolvadex.pro/# liquid tamoxifen
Top 100 Searched Drugs http://zithromaxpharm.online/# zithromax cost
zithromax online australia: zithromax buy – zithromax canadian pharmacy
https://clomidpharm.shop/# where to buy clomid prices
can you get generic clomid without dr prescription get generic clomid without prescription can you buy generic clomid for sale
http://clomidpharm.shop/# where can i get generic clomid no prescription
Their commitment to healthcare excellence is evident http://zithromaxpharm.online/# zithromax price south africa
nolvadex generic: tamoxifen skin changes – tamoxifen lawsuit
A name synonymous with international pharmaceutical trust https://clomidpharm.shop/# where to get cheap clomid price
http://clomidpharm.shop/# where to get cheap clomid for sale
effexor and tamoxifen: alternatives to tamoxifen – does tamoxifen cause bone loss
They make international medication sourcing a breeze https://cytotec.directory/# cytotec pills buy online
http://cytotec.directory/# buy cytotec
https://nolvadex.pro/# liquid tamoxifen
prednisone drug costs prednisone 25mg from canada prednisone 5443
buy zithromax without presc: zithromax 500mg – can i buy zithromax online
https://prednisonepharm.store/# generic prednisone pills
They ensure global standards in every pill https://zithromaxpharm.online/# zithromax coupon
prednisone buy: prednisone – prednisone 10mg tabs
Always stocked with what I need https://prednisonepharm.store/# prednisone 40 mg
https://nolvadex.pro/# nolvadex pct
where to get zithromax over the counter: purchase zithromax online – order zithromax without prescription
buy zithromax online with mastercard zithromax online australia zithromax buy online no prescription
Their loyalty program offers great deals https://cytotec.directory/# order cytotec online
https://clomidpharm.shop/# order generic clomid without rx
https://zithromaxpharm.online/# zithromax prescription online
Misoprostol 200 mg buy online: buy cytotec over the counter – buy cytotec online
Quick service without compromising on quality http://clomidpharm.shop/# can you get clomid without a prescription
https://edwithoutdoctorprescription.store/# viagra without a doctor prescription
canadian pharmacieswith no prescription: buy prescription drugs canada – my canadian family pharmacy
meds online without doctor prescription sildenafil without a doctor’s prescription buy prescription drugs without doctor
best erectile dysfunction pills ed treatment review medicine for erectile
buy prescription drugs online: non prescription erection pills – prescription drugs online without doctor
erection pills online: best ed pills – cheap erectile dysfunction
http://edpills.bid/# erectile dysfunction medicines
https://edwithoutdoctorprescription.store/# legal to buy prescription drugs from canada
cheap rx drugs canadian pharmacy store real canadian pharmacy
my canadian family pharmacy http://reputablepharmacies.online/# canadian pharmacy usa
list of 24 hour pharmacies
buying drugs canada: tadalafil canadian pharmacy – most reliable online pharmacies
http://edpills.bid/# ed meds online
non prescription ed drugs non prescription ed pills viagra without a doctor prescription
non prescription ed drugs: legal to buy prescription drugs from canada – viagra without doctor prescription
https://reputablepharmacies.online/# buy medicine canada
best canadian mail order pharmacy online pharmacy reviews cheap canadian cialis
canadian pharmacy generic viagra: mexican pharmacies that ship – canada pharmacy online canada pharmacies
cialis without doctor prescription: viagra without a doctor prescription – prescription drugs without prior prescription
prescription drugs online without doctor best ed pills non prescription buy prescription drugs from canada
canadian drugs https://reputablepharmacies.online/# canadian pharmacy online no prescription
thecanadianpharmacy com
http://reputablepharmacies.online/# prescription prices comparison
https://edpills.bid/# generic ed drugs
cheap erectile dysfunction pill: treatments for ed – ed pills gnc
top ed pills ed drugs list best pills for ed
https://edpills.bid/# ed medication
canadian discount cialis canadian pharcharmy canadian pharmaceutical companies that ship to usa
non prescription medicine pharmacy: reputable canadian mail order pharmacy – certified canadian pharmacy
https://reputablepharmacies.online/# bestpharmacyonline.com
cheapest ed pills: pills erectile dysfunction – erection pills
non prescription ed pills mexican pharmacy without prescription real viagra without a doctor prescription
best ed pills non prescription: gnc ed pills – ed treatment pills
https://canadianpharmacy.pro/# canadian pharmacy no rx needed canadianpharmacy.pro
canadian pharmacy reviews: Canadian pharmacy online – canadian pharmacy no scripts canadianpharmacy.pro
recommended canadian pharmacies Canadian pharmacy online best online canadian pharmacy canadianpharmacy.pro
mexican drugstore online Medicines Mexico mexico drug stores pharmacies mexicanpharmacy.win
best online pharmacies in mexico: mexican drugstore online – buying prescription drugs in mexico mexicanpharmacy.win
http://indianpharmacy.shop/# top 10 pharmacies in india indianpharmacy.shop
canadian pharmacy uk delivery Canada Pharmacy canadian online pharmacy canadianpharmacy.pro
buy medicines online in india: international medicine delivery from india – india online pharmacy indianpharmacy.shop
http://canadianpharmacy.pro/# canadian drugs online canadianpharmacy.pro
http://mexicanpharmacy.win/# mexican pharmaceuticals online mexicanpharmacy.win
https://canadianpharmacy.pro/# buy prescription drugs from canada cheap canadianpharmacy.pro
cheap meds no prescription
mexican pharmacy Medicines Mexico mexican pharmacy mexicanpharmacy.win
canadian world pharmacy: pharmacy rx world canada – canadian pharmacy tampa canadianpharmacy.pro
http://canadianpharmacy.pro/# best canadian pharmacy to buy from canadianpharmacy.pro
canada pharmacy reviews Canada Pharmacy canadian drug prices canadianpharmacy.pro
http://mexicanpharmacy.win/# mexican pharmacy mexicanpharmacy.win
india pharmacy mail order international medicine delivery from india indian pharmacies safe indianpharmacy.shop
http://mexicanpharmacy.win/# mexican border pharmacies shipping to usa mexicanpharmacy.win
Online medicine home delivery
india pharmacy Order medicine from India to USA indian pharmacies safe indianpharmacy.shop
https://canadianpharmacy.pro/# pharmacy rx world canada canadianpharmacy.pro
https://canadianpharmacy.pro/# pharmacy canadian canadianpharmacy.pro
https://indianpharmacy.shop/# pharmacy website india indianpharmacy.shop
canada medicine
http://indianpharmacy.shop/# indian pharmacy online indianpharmacy.shop
india online pharmacy
Online medicine order international medicine delivery from india top 10 online pharmacy in india indianpharmacy.shop
https://indianpharmacy.shop/# top online pharmacy india indianpharmacy.shop
https://indianpharmacy.shop/# india pharmacy indianpharmacy.shop
indian pharmacy
top online pharmacy india Cheapest online pharmacy indian pharmacies safe indianpharmacy.shop
http://indianpharmacy.shop/# best india pharmacy indianpharmacy.shop
http://mexicanpharmacy.win/# pharmacies in mexico that ship to usa mexicanpharmacy.win
mail order pharmacy india
mexico drug stores pharmacies Mexico pharmacy п»їbest mexican online pharmacies mexicanpharmacy.win
http://indianpharmacy.shop/# india pharmacy indianpharmacy.shop
http://canadianpharmacy.pro/# onlinepharmaciescanada com canadianpharmacy.pro
top 10 online pharmacy in india
indianpharmacy com Best Indian pharmacy top 10 pharmacies in india indianpharmacy.shop
http://mexicanpharmacy.win/# buying from online mexican pharmacy mexicanpharmacy.win
https://mexicanpharmacy.win/# best online pharmacies in mexico mexicanpharmacy.win
mail order pharmacy india
online shopping pharmacy india indian pharmacy buy prescription drugs from india indianpharmacy.shop
https://mexicanpharmacy.win/# buying from online mexican pharmacy mexicanpharmacy.win
http://indianpharmacy.shop/# indian pharmacies safe indianpharmacy.shop
http://indianpharmacy.shop/# indian pharmacies safe indianpharmacy.shop
india online pharmacy
п»їpharmacie en ligne: pharmacie en ligne sans ordonnance – Acheter mГ©dicaments sans ordonnance sur internet
Pharmacie en ligne France levitra generique prix en pharmacie acheter mГ©dicaments Г l’Г©tranger
http://acheterkamagra.pro/# acheter médicaments à l’étranger
п»їpharmacie en ligne: Medicaments en ligne livres en 24h – Acheter mГ©dicaments sans ordonnance sur internet
Pharmacie en ligne livraison rapide Acheter Cialis 20 mg pas cher Pharmacie en ligne pas cher
http://acheterkamagra.pro/# pharmacie ouverte 24/24
Pharmacie en ligne livraison 24h
http://pharmadoc.pro/# Pharmacie en ligne fiable
Pharmacie en ligne livraison rapide: kamagra 100mg prix – pharmacie ouverte 24/24
acheter mГ©dicaments Г l’Г©tranger Pharmacie en ligne sans ordonnance Pharmacie en ligne pas cher
pharmacie ouverte 24/24: Pharmacies en ligne certifiГ©es – acheter mГ©dicaments Г l’Г©tranger
https://viagrasansordonnance.pro/# Sildénafil 100 mg sans ordonnance
Pharmacie en ligne pas cher: levitra generique sites surs – Pharmacie en ligne fiable
Pharmacie en ligne pas cher Pharmacies en ligne certifiees Pharmacie en ligne livraison gratuite
http://viagrasansordonnance.pro/# Prix du Viagra 100mg en France
Pharmacie en ligne livraison 24h: levitra generique sites surs – pharmacie ouverte 24/24
http://pharmadoc.pro/# pharmacie ouverte
Acheter mГ©dicaments sans ordonnance sur internet
https://acheterkamagra.pro/# Pharmacie en ligne sans ordonnance
pharmacie ouverte: kamagra gel – Pharmacie en ligne sans ordonnance
Pharmacie en ligne livraison rapide: PharmaDoc – Pharmacie en ligne livraison 24h
Pharmacie en ligne pas cher: Cialis sans ordonnance 24h – pharmacie ouverte
Pharmacie en ligne livraison rapide cialissansordonnance.shop pharmacie ouverte 24/24
Pharmacie en ligne sans ordonnance: Levitra pharmacie en ligne – pharmacie ouverte
Pharmacie en ligne livraison 24h Acheter Cialis Pharmacie en ligne livraison gratuite
prednisone 5443: cheapest prednisone no prescription – buy prednisone without rx
zithromax price south africa where can i get zithromax can i buy zithromax online
http://prednisonetablets.shop/# how can i get prednisone
cost of cheap clomid tablets: can you get generic clomid – buying cheap clomid without insurance
stromectol pills ivermectin oral 0 8 ivermectin canada
stromectol covid: stromectol uk buy – ivermectin cost uk
http://clomiphene.icu/# how to get clomid without insurance
amoxicillin 500mg price: amoxicillin online purchase – buy amoxicillin over the counter uk
brand prednisone prednisone 20mg prescription cost buy prednisone canada
http://ivermectin.store/# stromectol coronavirus
can i purchase generic clomid without dr prescription: can you buy cheap clomid without dr prescription – can i order clomid online
prednisone 5 50mg tablet price 5 mg prednisone tablets prednisone without a prescription
http://ivermectin.store/# stromectol tablet 3 mg
ivermectin 1: ivermectin 0.08 oral solution – buy oral ivermectin
zithromax online australia: buy zithromax without prescription online – zithromax online usa no prescription
https://prednisonetablets.shop/# iv prednisone
buy amoxicillin canada amoxicillin 500mg capsules antibiotic amoxicillin buy canada
ivermectin cream canada cost: ivermectin buy online – stromectol drug
http://amoxicillin.bid/# buy amoxicillin 500mg uk
http://azithromycin.bid/# can i buy zithromax online
where to buy prednisone 20mg no prescription: buy generic prednisone online – how can i order prednisone
prednisone 50 mg buy prednisone nz prednisone 20 mg tablets
prescription for amoxicillin: amoxicillin 500mg prescription – can you buy amoxicillin over the counter
https://ivermectin.store/# ivermectin 3mg dosage
prednisone 1 mg daily: prednisone pill 20 mg – 5 mg prednisone daily
buy zithromax online cheap zithromax online australia zithromax generic cost
20 mg prednisone: 1 mg prednisone daily – prednisone 2.5 mg daily
http://ivermectin.store/# ivermectin cream 5%
http://amoxicillin.bid/# amoxicillin medicine
amoxicillin azithromycin amoxicillin 500 mg capsule amoxicillin 500 mg cost
amoxicillin buy no prescription: amoxicillin 500 mg without a prescription – amoxicillin without rx
prednisone in india: order prednisone 10 mg tablet – canada pharmacy prednisone
http://prednisonetablets.shop/# iv prednisone
indian pharmacies safe: order medicine from india to usa – top 10 pharmacies in india indianpharm.store
indian pharmacies safe top 10 online pharmacy in india Online medicine home delivery indianpharm.store
pharmacy website india: Indian pharmacy to USA – india online pharmacy indianpharm.store
canadian pharmacy uk delivery: Canadian International Pharmacy – ed meds online canada canadianpharm.store
mexican mail order pharmacies Online Pharmacies in Mexico mexican border pharmacies shipping to usa mexicanpharm.shop
https://indianpharm.store/# reputable indian pharmacies indianpharm.store
reputable indian online pharmacy: Indian pharmacy to USA – reputable indian pharmacies indianpharm.store
mexican drugstore online: Online Mexican pharmacy – mexico pharmacies prescription drugs mexicanpharm.shop
canadian pharmacy online store Pharmacies in Canada that ship to the US best canadian pharmacy canadianpharm.store
http://canadianpharm.store/# best canadian pharmacy canadianpharm.store
https://indianpharm.store/# Online medicine order indianpharm.store
medicine in mexico pharmacies: purple pharmacy mexico price list – best online pharmacies in mexico mexicanpharm.shop
http://indianpharm.store/# india online pharmacy indianpharm.store
indian pharmacies safe: cheapest online pharmacy india – indian pharmacy paypal indianpharm.store
thecanadianpharmacy Certified Online Pharmacy Canada canada pharmacy canadianpharm.store
ed meds online canada: Pharmacies in Canada that ship to the US – canadian pharmacy no scripts canadianpharm.store
https://indianpharm.store/# online shopping pharmacy india indianpharm.store
canadian pharmacy victoza: Canadian Pharmacy – canada cloud pharmacy canadianpharm.store
buying from canadian pharmacies Canadian Pharmacy canadian pharmacy uk delivery canadianpharm.store
legal canadian pharmacy online: Certified Online Pharmacy Canada – canadian pharmacy online store canadianpharm.store
https://mexicanpharm.shop/# mexican drugstore online mexicanpharm.shop
mexico drug stores pharmacies: mexican mail order pharmacies – buying from online mexican pharmacy mexicanpharm.shop
best india pharmacy best india pharmacy india pharmacy indianpharm.store
http://mexicanpharm.shop/# mexico drug stores pharmacies mexicanpharm.shop
http://indianpharm.store/# buy prescription drugs from india indianpharm.store
Online medicine home delivery: top 10 online pharmacy in india – pharmacy website india indianpharm.store
canada ed drugs: Canada Pharmacy online – canada drug pharmacy canadianpharm.store
http://indianpharm.store/# buy medicines online in india indianpharm.store
medication from mexico pharmacy mexico drug stores pharmacies buying prescription drugs in mexico online mexicanpharm.shop
reputable canadian pharmacy: Canadian Pharmacy – best canadian pharmacy to buy from canadianpharm.store
canadian pharmacy in canada: Canada Pharmacy online – canadian pharmacy canadianpharm.store
mexico pharmacy: Online Pharmacies in Mexico – mexico pharmacies prescription drugs mexicanpharm.shop
https://indianpharm.store/# indian pharmacy paypal indianpharm.store
mexican border pharmacies shipping to usa Online Pharmacies in Mexico medicine in mexico pharmacies mexicanpharm.shop
cheapest online pharmacy india: indian pharmacy paypal – cheapest online pharmacy india indianpharm.store
http://canadianpharm.store/# canadian valley pharmacy canadianpharm.store
http://mexicanpharm.shop/# mexican rx online mexicanpharm.shop
reputable indian pharmacies: international medicine delivery from india – indian pharmacy online indianpharm.store
77 canadian pharmacy Licensed Online Pharmacy drugs from canada canadianpharm.store
online shopping pharmacy india: indian pharmacies safe – top 10 pharmacies in india indianpharm.store
http://indianpharm.store/# indian pharmacy online indianpharm.store
canadian pharmacy in canada: Canadian International Pharmacy – reliable canadian pharmacy reviews canadianpharm.store
indianpharmacy com international medicine delivery from india top 10 pharmacies in india indianpharm.store
canadian pharmacy: Licensed Online Pharmacy – vipps approved canadian online pharmacy canadianpharm.store
https://mexicanpharm.shop/# buying prescription drugs in mexico online mexicanpharm.shop
top 10 online pharmacy in india order medicine from india to usa india pharmacy indianpharm.store
http://canadianpharm.store/# best canadian pharmacy online canadianpharm.store
mexican online pharmacies prescription drugs: mexico drug stores pharmacies – mexico pharmacies prescription drugs mexicanpharm.shop
mexican rx online: Certified Pharmacy from Mexico – mexico pharmacies prescription drugs mexicanpharm.shop
https://indianpharm.store/# cheapest online pharmacy india indianpharm.store
internet pharmacy no prescription: canadian pharmacy products – bestpharmacyonline.com
legitimate canadian pharmacies compare medication prices top rated online pharmacy
https://canadadrugs.pro/# canadian pharmacy online
cost prescription drugs: best online drugstore – canadian pharmacy non prescription
top 10 online pharmacies: overseas pharmacies – cheap canadian cialis
mexican online pharmacy drugs from canada without prescription canadian pharmacies without an rx
https://canadadrugs.pro/# canadian drugs pharmacies online
canadian online pharmacies legitimate by aarp: drugs without a doctor s prescription – prescription drug pricing
legitimate canadian mail order pharmacy: online pharmacy no scripts – canadian drug store viagra
get canadian drugs online meds no rx reliable price prescriptions
https://canadadrugs.pro/# canadian pharmacy no presciption
best online canadian pharmacy review: nabp canadian pharmacy – canadian mail order drug companies
canadian pharmacy store: online meds no rx reliable – reputable online canadian pharmacies
http://canadadrugs.pro/# top rated online canadian pharmacies
drugs without a prescription online pharmacies in usa best canadian pharmacies
canada pharmacies online prescriptions: canadian drug – prescription drugs canadian
https://canadadrugs.pro/# canadian pharmacy review
canadian pharmacy no prescription required mexican pharmacy drugs canadian online pharmacies legitimate by aarp
best online pharmacy no prescription: legitimate online pharmacies – online canadian discount pharmacy
canadian pharmacy 24hr: canadian pharmacies shipping to usa – most reliable online pharmacies
http://canadadrugs.pro/# canadian online pharmacies legitimate by aarp
best 10 online pharmacies: ed meds without doctor prescription – online pharmacy
safe reliable canadian pharmacy: canadian internet pharmacy – prescription drugs online
https://canadadrugs.pro/# reliable online pharmacies
best mail order canadian pharmacy: giant discount pharmacy – reputable canadian pharmacy online
good online mexican pharmacy: legit canadian pharmacy online – ed meds online without doctor prescription
http://canadadrugs.pro/# canada pharmacy
best canadian online pharmacy: best 10 online pharmacies – canadian prescription drugs online
http://canadadrugs.pro/# online pharmacy medications
canada prescription: canadian pharmacy no prescription required – best canadian prescription prices
canadian drug stores: no prior prescription needed – mail order pharmacies
legal canadian prescription drugs online: canadian pharmacy online no prescription – canada pharmaceutical online ordering
https://canadadrugs.pro/# top online pharmacies
http://canadadrugs.pro/# canadian drug companies
discount pharmacy coupons mexican mail order pharmacy reliable online pharmacies
list of canadian pharmacies online: top rated canadian mail order pharmacies – drugs from canada with prescription
buying from online mexican pharmacy: medication from mexico pharmacy – best online pharmacies in mexico
top online pharmacy india indianpharmacy com indianpharmacy com
https://medicinefromindia.store/# Online medicine order
legal to buy prescription drugs from canada: cheap cialis – prescription drugs online without doctor
mexican pharmaceuticals online mexican pharmacy mexican pharmaceuticals online
http://edwithoutdoctorprescription.pro/# viagra without doctor prescription amazon
ed meds online without doctor prescription: cheap cialis – non prescription ed drugs
buy erection pills: top ed drugs – generic ed pills
pharmacy website india online shopping pharmacy india indian pharmacies safe
https://canadianinternationalpharmacy.pro/# canada drug pharmacy
http://edwithoutdoctorprescription.pro/# buy prescription drugs from canada
india online pharmacy india online pharmacy indian pharmacy online
https://medicinefromindia.store/# legitimate online pharmacies india
canada cloud pharmacy: best canadian pharmacy – canada online pharmacy
https://edwithoutdoctorprescription.pro/# prescription drugs without doctor approval
canadian discount pharmacy canadian pharmacies that deliver to the us best canadian pharmacy
best online pharmacy india: world pharmacy india – Online medicine order
https://certifiedpharmacymexico.pro/# pharmacies in mexico that ship to usa
reputable indian online pharmacy pharmacy website india top 10 pharmacies in india
http://edpill.cheap/# best ed pills online
medications for ed: best ed pills non prescription – gnc ed pills
mexican border pharmacies shipping to usa medicine in mexico pharmacies mexican rx online
https://edwithoutdoctorprescription.pro/# online prescription for ed meds
prescription drugs canada buy online: cialis without a doctor prescription canada – cialis without a doctor’s prescription
india pharmacy mail order mail order pharmacy india best online pharmacy india
http://edpill.cheap/# erection pills
http://certifiedpharmacymexico.pro/# best online pharmacies in mexico
mexican pharmacy mexican mail order pharmacies mexico pharmacies prescription drugs
https://certifiedpharmacymexico.pro/# best online pharmacies in mexico
best non prescription ed pills medicine for impotence erection pills viagra online
pharmacies in mexico that ship to usa: purple pharmacy mexico price list – mexican border pharmacies shipping to usa
http://medicinefromindia.store/# legitimate online pharmacies india
mexico drug stores pharmacies п»їbest mexican online pharmacies mexico pharmacy
https://canadianinternationalpharmacy.pro/# canadian pharmacy 24 com
mexico pharmacy mexico drug stores pharmacies mexico pharmacy
http://canadianinternationalpharmacy.pro/# canadian mail order pharmacy
generic viagra without a doctor prescription: buy cheap prescription drugs online – buy prescription drugs online without
https://canadianinternationalpharmacy.pro/# vipps approved canadian online pharmacy
certified canadian international pharmacy canada drugs reviews prescription drugs canada buy online
https://medicinefromindia.store/# Online medicine home delivery
п»їprescription drugs generic cialis without a doctor prescription buy prescription drugs online without
http://medicinefromindia.store/# india pharmacy mail order
http://medicinefromindia.store/# india pharmacy mail order
mexican mail order pharmacies buying prescription drugs in mexico п»їbest mexican online pharmacies
prescription drugs online without doctor: cialis without a doctor prescription – viagra without doctor prescription
http://canadianinternationalpharmacy.pro/# canadian medications
mexican mail order pharmacies mexico pharmacies prescription drugs purple pharmacy mexico price list
https://canadianinternationalpharmacy.pro/# legitimate canadian pharmacies
canadian pharmacy online reviews my canadian pharmacy reviews canadian drug pharmacy
https://canadianinternationalpharmacy.pro/# safe canadian pharmacy
best erection pills non prescription erection pills best non prescription ed pills
http://canadianinternationalpharmacy.pro/# rate canadian pharmacies
best ed pills non prescription: natural ed medications – erection pills online
mexican online pharmacies prescription drugs mexican pharmacy buying prescription drugs in mexico
medicine in mexico pharmacies medication from mexico pharmacy best mexican online pharmacies
reputable mexican pharmacies online medication from mexico pharmacy mexican pharmaceuticals online
http://mexicanph.com/# best online pharmacies in mexico
medication from mexico pharmacy
mexican rx online medication from mexico pharmacy mexican rx online
mexican mail order pharmacies mexico drug stores pharmacies mexican rx online
mexican mail order pharmacies buying prescription drugs in mexico online best online pharmacies in mexico
mexican rx online reputable mexican pharmacies online purple pharmacy mexico price list
mexico pharmacy mexican pharmacy best mexican online pharmacies
mexican online pharmacies prescription drugs best online pharmacies in mexico mexican online pharmacies prescription drugs
mexico pharmacy mexican border pharmacies shipping to usa best online pharmacies in mexico
https://mexicanph.shop/# mexican border pharmacies shipping to usa
mexico pharmacies prescription drugs
medication from mexico pharmacy mexico drug stores pharmacies mexico pharmacy
mexican border pharmacies shipping to usa reputable mexican pharmacies online buying from online mexican pharmacy
mexican pharmacy mexico pharmacies prescription drugs best online pharmacies in mexico
mexican pharmacy medication from mexico pharmacy п»їbest mexican online pharmacies
https://mexicanph.shop/# п»їbest mexican online pharmacies
mexican rx online
mexican rx online medication from mexico pharmacy mexico drug stores pharmacies
mexican pharmaceuticals online mexican border pharmacies shipping to usa mexico pharmacies prescription drugs
reputable mexican pharmacies online medication from mexico pharmacy mexico pharmacies prescription drugs
https://mexicanph.shop/# buying prescription drugs in mexico
mexican mail order pharmacies
mexican rx online п»їbest mexican online pharmacies buying from online mexican pharmacy
buying prescription drugs in mexico online reputable mexican pharmacies online mexican rx online
buying from online mexican pharmacy buying prescription drugs in mexico mexican border pharmacies shipping to usa
buying prescription drugs in mexico online mexican mail order pharmacies mexican mail order pharmacies
medicine in mexico pharmacies medicine in mexico pharmacies mexican pharmaceuticals online
mexico pharmacy pharmacies in mexico that ship to usa medication from mexico pharmacy
mexico drug stores pharmacies buying from online mexican pharmacy mexico pharmacies prescription drugs
buying prescription drugs in mexico best online pharmacies in mexico buying from online mexican pharmacy
buying from online mexican pharmacy mexican mail order pharmacies best mexican online pharmacies
zithromax during pregnancy
http://mexicanph.com/# pharmacies in mexico that ship to usa
п»їbest mexican online pharmacies
mexican online pharmacies prescription drugs best online pharmacies in mexico mexican drugstore online
buying from online mexican pharmacy reputable mexican pharmacies online mexican mail order pharmacies
mexican drugstore online mexican rx online mexican online pharmacies prescription drugs
mexican pharmaceuticals online mexican border pharmacies shipping to usa mexico pharmacies prescription drugs
reputable mexican pharmacies online pharmacies in mexico that ship to usa mexican border pharmacies shipping to usa
mexico drug stores pharmacies buying prescription drugs in mexico medication from mexico pharmacy
purple pharmacy mexico price list medicine in mexico pharmacies mexican pharmacy
reputable mexican pharmacies online mexican drugstore online mexican drugstore online
п»їbest mexican online pharmacies mexican drugstore online mexican online pharmacies prescription drugs
mexico pharmacy mexico drug stores pharmacies mexico pharmacies prescription drugs
https://mexicanph.shop/# pharmacies in mexico that ship to usa
buying prescription drugs in mexico
best online pharmacies in mexico buying prescription drugs in mexico purple pharmacy mexico price list
mexican border pharmacies shipping to usa mexican online pharmacies prescription drugs mexico drug stores pharmacies
buying from online mexican pharmacy mexico pharmacies prescription drugs pharmacies in mexico that ship to usa
mexican rx online buying from online mexican pharmacy mexican pharmacy
mexico drug stores pharmacies medicine in mexico pharmacies mexico drug stores pharmacies
buying from online mexican pharmacy mexican pharmacy best mexican online pharmacies
п»їbest mexican online pharmacies reputable mexican pharmacies online mexico pharmacies prescription drugs
mexican pharmaceuticals online pharmacies in mexico that ship to usa buying prescription drugs in mexico
best online pharmacies in mexico mexico pharmacy best online pharmacies in mexico
purple pharmacy mexico price list mexico pharmacies prescription drugs mexican pharmacy
buying prescription drugs in mexico online mexican online pharmacies prescription drugs mexican drugstore online
best mexican online pharmacies best online pharmacies in mexico mexico pharmacies prescription drugs
best online pharmacies in mexico pharmacies in mexico that ship to usa buying prescription drugs in mexico online
buying prescription drugs in mexico online mexico pharmacy pharmacies in mexico that ship to usa
mexican pharmaceuticals online mexico drug stores pharmacies pharmacies in mexico that ship to usa
http://mexicanph.shop/# mexico drug stores pharmacies
mexico pharmacies prescription drugs
reputable mexican pharmacies online mexico pharmacies prescription drugs purple pharmacy mexico price list
pharmacies in mexico that ship to usa buying from online mexican pharmacy mexican pharmacy
mexican drugstore online п»їbest mexican online pharmacies mexican border pharmacies shipping to usa
mexican rx online mexican border pharmacies shipping to usa purple pharmacy mexico price list
п»їbest mexican online pharmacies pharmacies in mexico that ship to usa mexico drug stores pharmacies
mexican drugstore online medication from mexico pharmacy mexican border pharmacies shipping to usa
buying prescription drugs in mexico best mexican online pharmacies mexican drugstore online
best online pharmacies in mexico mexican mail order pharmacies purple pharmacy mexico price list
п»їbest mexican online pharmacies medication from mexico pharmacy mexican rx online
best online pharmacies in mexico best online pharmacies in mexico mexican drugstore online
buying prescription drugs in mexico online mexican border pharmacies shipping to usa mexican border pharmacies shipping to usa
buying prescription drugs in mexico online buying from online mexican pharmacy mexican online pharmacies prescription drugs
mexico drug stores pharmacies mexico pharmacy best online pharmacies in mexico
mexico pharmacies prescription drugs pharmacies in mexico that ship to usa mexican border pharmacies shipping to usa
mexican online pharmacies prescription drugs mexican online pharmacies prescription drugs pharmacies in mexico that ship to usa
mexican pharmaceuticals online mexican border pharmacies shipping to usa pharmacies in mexico that ship to usa
mexican rx online mexican mail order pharmacies mexican mail order pharmacies
buying prescription drugs in mexico online medication from mexico pharmacy mexico drug stores pharmacies
buying from online mexican pharmacy mexican pharmaceuticals online purple pharmacy mexico price list
best online pharmacies in mexico reputable mexican pharmacies online mexican pharmacy
medicine in mexico pharmacies mexico pharmacy mexico drug stores pharmacies
http://mexicanph.com/# mexican online pharmacies prescription drugs
mexican online pharmacies prescription drugs
mexican rx online pharmacies in mexico that ship to usa mexico pharmacy
prednisone otc price: prednisone steroids – prednisone 50 mg coupon
lasix 100 mg tablet Over The Counter Lasix lasix online
https://buyprednisone.store/# prednisone 21 pack
stromectol tab: stromectol otc – ivermectin malaria
http://furosemide.guru/# furosemide 100 mg
lasix uses: Buy Lasix No Prescription – lasix medication
lasix uses Buy Lasix generic lasix
https://stromectol.fun/# ivermectin 90 mg
https://buyprednisone.store/# over the counter prednisone cream
lasix online: Buy Lasix No Prescription – lasix uses
https://amoxil.cheap/# amoxicillin 500mg pill
208 lisinopril: lisinopril generic 10 mg – lisinopril buy in canada
generic over the counter prednisone buy prednisone 20mg without a prescription best price prednisone 2.5 mg cost
stromectol for sale: buy ivermectin canada – ivermectin 200mg
https://buyprednisone.store/# prednisone generic brand name
http://stromectol.fun/# stromectol covid 19
buy amoxicillin without prescription amoxicillin 500 where can i buy amoxicillin over the counter uk
http://amoxil.cheap/# buy amoxicillin 500mg
order amoxicillin online uk: cost of amoxicillin – can i buy amoxicillin over the counter
furosemida 40 mg: Buy Lasix – generic lasix
https://furosemide.guru/# buy furosemide online
lasix 20 mg: Buy Lasix No Prescription – furosemida
over the counter prednisone medicine prednisone prescription drug prednisone daily
http://lisinopril.top/# lisinopril 15 mg
amoxicillin discount coupon: can i buy amoxicillin over the counter in australia – amoxicillin without a prescription
prednisone 5 50mg tablet price: prednisone pill – prednisone coupon
https://stromectol.fun/# ivermectin iv
ivermectin cost stromectol tablets buy online stromectol ireland
purchase lisinopril 40 mg: can i buy lisinopril over the counter in mexico – can you order lisinopril online
http://furosemide.guru/# lasix uses
https://stromectol.fun/# cost of ivermectin medicine
prednisone 1 mg daily: 1 mg prednisone daily – prednisone cost canada
lisinopril 5 mg: lisinopril over the counter – lisinopril 20mg online
https://buyprednisone.store/# prednisone 50 mg coupon
cheap amoxicillin 500mg amoxicillin 500mg capsule buy online amoxicillin order online
http://amoxil.cheap/# order amoxicillin online
ivermectin 1 topical cream: ivermectin 3 mg – ivermectin 6 mg tablets
http://buyprednisone.store/# prednisone 20mg prices
buy prednisone from india: buy prednisone nz – how much is prednisone 10 mg
rx lisinopril: zestril canada – lisinopril 2
lasix dosage Buy Lasix furosemida
https://lisinopril.top/# buy lisinopril 10 mg online
https://furosemide.guru/# lasix 40mg
amoxicillin 500 mg for sale: amoxicillin 875 mg tablet – cheap amoxicillin 500mg
https://furosemide.guru/# lasix 100mg
amoxicillin pills 500 mg: buy amoxicillin 500mg uk – amoxicillin 500 mg capsule
prednisone 5084 20mg prednisone prednisone price canada
buy stromectol uk: ivermectin ireland – ivermectin virus
https://furosemide.guru/# furosemide 100mg
http://lisinopril.top/# lisinopril 30 mg tablet
how to buy amoxicillin online: amoxicillin discount coupon – where can i buy amoxocillin
lasix 40 mg Buy Lasix No Prescription furosemida
furosemida: Buy Furosemide – lasix side effects
https://amoxil.cheap/# amoxicillin 500mg over the counter
lasix generic name: Buy Lasix – lasix medication
https://furosemide.guru/# lasix for sale
http://buyprednisone.store/# generic prednisone cost
prednisone 40 mg daily: generic prednisone otc – prednisone 5 mg tablet price
https://stromectol.fun/# ivermectin human
amoxicillin without prescription: amoxicillin 500mg prescription – amoxicillin 500 mg online
https://buyprednisone.store/# buy prednisone 10mg online
cost of ivermectin medicine: stromectol buy – ivermectin tablets order
prednisone price south africa prednisone no rx prednisone 10mg tablets
http://buyprednisone.store/# prednisone tablets
lisinopril 20 mg online: buy lisinopril 2.5 mg – buy lisinopril 10 mg online
lisinopril pill 20mg: zestril 2.5 mg – prinivil lisinopril
https://stromectol.fun/# ivermectin cream uk
http://lisinopril.top/# lisinopril 80
stromectol tab 3mg ivermectin pills human stromectol tab price
https://stromectol.fun/# ivermectin buy online
generic lasix: Buy Lasix – lasix 40mg
lisinopril generic brand: lisinopril 2.5 – buy lisinopril 20 mg
http://buyprednisone.store/# 20 mg of prednisone
ivermectin 1 cream generic: ivermectin india – stromectol usa
prednisone 10 mg over the counter buy prednisone online uk prednisone 50 mg coupon
https://amoxil.cheap/# generic amoxicillin
http://furosemide.guru/# furosemide 40 mg
ivermectin stromectol: ivermectin 6 tablet – ivermectin virus
buy cheap amoxicillin: amoxicillin tablets in india – amoxicillin 500 mg purchase without prescription
http://stromectol.fun/# stromectol generic name
generic name for ivermectin ivermectin 3 mg dose cost of stromectol
http://buyprednisone.store/# where to buy prednisone in canada
lisinopril 5mg: lisinopril canada – zestril 30 mg
http://lisinopril.top/# lisinopril with out prescription
lasix 40 mg: Buy Furosemide – furosemida 40 mg
ivermectin 200mg: stromectol covid – ivermectin tablets uk
http://buyprednisone.store/# prednisone 50 mg price
amoxicillin 500 amoxicillin discount amoxicillin 775 mg
https://stromectol.fun/# ivermectin 3mg pill
generic lasix: Buy Lasix No Prescription – lasix pills
cheapest online pharmacy india reputable indian online pharmacy indianpharmacy com
http://indianph.xyz/# pharmacy website india
top online pharmacy india
http://indianph.xyz/# india pharmacy mail order
Online medicine home delivery
top 10 pharmacies in india india online pharmacy buy medicines online in india
india pharmacy mail order indian pharmacy paypal india pharmacy
https://indianph.com/# india pharmacy
http://indianph.xyz/# top 10 pharmacies in india
reputable indian pharmacies
http://indianph.com/# indianpharmacy com
india pharmacy
online shopping pharmacy india online pharmacy india mail order pharmacy india
Online medicine order indian pharmacy india pharmacy mail order
https://indianph.xyz/# online pharmacy india
top 10 pharmacies in india
https://indianph.xyz/# best online pharmacy india
best india pharmacy
https://indianph.xyz/# п»їlegitimate online pharmacies india
https://indianph.xyz/# pharmacy website india
pharmacy website india
buy medicines online in india pharmacy website india buy medicines online in india
buy cytotec over the counter buy cytotec buy cytotec pills
diflucan 2 pills: diflucan no prescription – buy diflucan online uk
https://diflucan.pro/# diflucan coupon canada
http://doxycycline.auction/# doxycycline 100mg
tamoxifen citrate pct: tamoxifen alternatives premenopausal – nolvadex vs clomid
diflucan cost in india where can you get diflucan over the counter diflucan tablet 500mg
https://cipro.guru/# ciprofloxacin mail online
http://cytotec24.shop/# cytotec online
tamoxifen 20 mg tablet: tamoxifen and antidepressants – tamoxifen for men
https://diflucan.pro/# fluconazole diflucan
purchase cytotec buy cytotec buy cytotec pills online cheap
http://cytotec24.shop/# purchase cytotec
cipro pharmacy: п»їcipro generic – ciprofloxacin 500 mg tablet price
https://doxycycline.auction/# doxycycline
cytotec buy online usa purchase cytotec buy cytotec pills
tamoxifen alternatives premenopausal: tamoxifen lawsuit – tamoxifen blood clots
https://cytotec24.shop/# cytotec pills online
https://nolvadex.guru/# nolvadex online
buy cipro cheap: buy cipro online canada – buy cipro online canada
http://nolvadex.guru/# tamoxifen and weight loss
diflucan 150 mg price in india buy diflucan 150mg purchase diflucan
http://nolvadex.guru/# tamoxifen and weight loss
https://nolvadex.guru/# natural alternatives to tamoxifen
should i take tamoxifen tamoxifen endometrium tamoxifen headache
http://cytotec24.shop/# buy cytotec online
https://cytotec24.com/# cytotec pills buy online
http://nolvadex.guru/# tamoxifen dosage
order cytotec online buy misoprostol over the counter buy cytotec in usa
http://cytotec24.com/# buy misoprostol over the counter
https://cytotec24.com/# buy cytotec online fast delivery
http://cipro.guru/# buy ciprofloxacin over the counter
buy doxycycline online without prescription buy doxycycline order doxycycline online
https://nolvadex.guru/# does tamoxifen make you tired
doxycycline medication doxycycline hyc 100mg doxycycline vibramycin
Angela White: abella danger izle – Abella Danger
https://lanarhoades.fun/# lana rhoades modeli
http://sweetiefox.online/# Sweetie Fox modeli
https://evaelfie.pro/# eva elfie video
swetie fox: swetie fox – swetie fox
https://angelawhite.pro/# Angela Beyaz modeli
https://lanarhoades.fun/# lana rhoades video
swetie fox: Sweetie Fox modeli – Sweetie Fox modeli
https://lanarhoades.fun/# lana rhoades modeli
https://angelawhite.pro/# Angela White filmleri
eva elfie filmleri: eva elfie video – eva elfie filmleri
https://evaelfie.pro/# eva elfie video
https://abelladanger.online/# Abella Danger
http://evaelfie.pro/# eva elfie filmleri
http://lanarhoades.fun/# lana rhoades izle
swetie fox: Sweetie Fox video – Sweetie Fox
https://evaelfie.pro/# eva elfie video
https://sweetiefox.online/# Sweetie Fox video
Sweetie Fox: Sweetie Fox filmleri – swetie fox
http://abelladanger.online/# abella danger izle
http://lanarhoades.fun/# lana rhoades izle
http://angelawhite.pro/# Angela White izle
lana rhoades filmleri: lana rhoades – lana rhoades izle
https://abelladanger.online/# abella danger izle
http://abelladanger.online/# Abella Danger
https://abelladanger.online/# Abella Danger
lana rhoades: lana rhoades modeli – lana rhoades filmleri
https://lanarhoades.fun/# lana rhoades filmleri
https://angelawhite.pro/# Angela White izle
https://lanarhoades.fun/# lana rhodes
https://lanarhoades.fun/# lana rhoades video
lana rhoades filmleri: lana rhoades filmleri – lana rhoades modeli
https://lanarhoades.fun/# lana rhoades modeli
https://angelawhite.pro/# ?????? ????
Sweetie Fox filmleri: Sweetie Fox modeli – Sweetie Fox modeli
http://lanarhoades.fun/# lana rhoades
https://sweetiefox.online/# Sweetie Fox filmleri
http://evaelfie.pro/# eva elfie modeli
https://evaelfie.pro/# eva elfie
Sweetie Fox modeli: swetie fox – Sweetie Fox
https://angelawhite.pro/# Angela White video
https://evaelfie.pro/# eva elfie video
Angela White: Angela White – Angela White izle
https://lanarhoades.fun/# lana rhoades izle
http://evaelfie.pro/# eva elfie filmleri
swetie fox: Sweetie Fox – sweeti fox
http://angelawhite.pro/# Angela White
https://angelawhite.pro/# Angela White
http://miamalkova.life/# mia malkova girl
eva elfie new video: eva elfie new video – eva elfie photo
sexy dating: http://sweetiefox.pro/# sweetie fox
http://evaelfie.site/# eva elfie full videos
eva elfie videos: eva elfie – eva elfie full video
eva elfie new video: eva elfie new video – eva elfie full videos
https://lanarhoades.pro/# lana rhoades solo
sweetie fox full: sweetie fox cosplay – sweetie fox cosplay
single senior dating site online: https://lanarhoades.pro/# lana rhoades hot
http://lanarhoades.pro/# lana rhoades hot
lana rhoades solo: lana rhoades full video – lana rhoades boyfriend
mia malkova only fans: mia malkova videos – mia malkova hd
http://lanarhoades.pro/# lana rhoades solo
lana rhoades full video: lana rhoades solo – lana rhoades unleashed
free online dating sites reviews: http://sweetiefox.pro/# ph sweetie fox
https://lanarhoades.pro/# lana rhoades videos
mia malkova full video: mia malkova full video – mia malkova photos
lana rhoades hot: lana rhoades solo – lana rhoades pics
sweetie fox: sweetie fox cosplay – fox sweetie
http://evaelfie.site/# eva elfie
best websites dating: https://lanarhoades.pro/# lana rhoades unleashed
eva elfie photo: eva elfie full videos – eva elfie full videos
http://sweetiefox.pro/# sweetie fox cosplay
eva elfie: eva elfie videos – eva elfie new videos
lana rhoades pics: lana rhoades full video – lana rhoades boyfriend
best sites online dating: http://miamalkova.life/# mia malkova latest
https://evaelfie.site/# eva elfie
eva elfie full videos: eva elfie hot – eva elfie videos
sweetie fox: sweetie fox full – sweetie fox full video
http://lanarhoades.pro/# lana rhoades
ph sweetie fox: sweetie fox new – sweetie fox full video
top dating sites: https://miamalkova.life/# mia malkova
http://sweetiefox.pro/# sweetie fox video
lana rhoades pics: lana rhoades solo – lana rhoades videos
http://sweetiefox.pro/# sweetie fox
sweetie fox full: sweetie fox – sweetie fox full
mia malkova latest: mia malkova latest – mia malkova latest
singles website for farmers: http://evaelfie.site/# eva elfie
site de apostas: site de apostas – jogos que dao dinheiro
https://aviatormocambique.site/# como jogar aviator
http://jogodeaposta.fun/# melhor jogo de aposta para ganhar dinheiro
aplicativo de aposta: melhor jogo de aposta para ganhar dinheiro – aviator jogo de aposta
http://aviatorghana.pro/# aviator betting game
melhor jogo de aposta: jogos que dão dinheiro – melhor jogo de aposta para ganhar dinheiro
pin up aviator: aviator oficial pin up – pin up aviator
aviator online: aviator online – aviator
https://aviatorghana.pro/# aviator ghana
http://aviatormocambique.site/# aviator mz
jogos que dão dinheiro: aplicativo de aposta – jogo de aposta online
https://jogodeaposta.fun/# melhor jogo de aposta
http://aviatormocambique.site/# aviator bet
http://aviatormocambique.site/# aviator online
aviator game: aviator bet – aviator
aviator: aviator game – aviator bet malawi login
como jogar aviator em moçambique: aviator bet – aviator
aviator betting game: aviator malawi – aviator bet
aviator bet malawi login: aviator bet malawi login – aviator bet malawi login
aviator game: aviator betting game – aviator malawi
https://pinupcassino.pro/# pin up aviator
ganhar dinheiro jogando: ganhar dinheiro jogando – jogos que dao dinheiro
aviator betting game: play aviator – aviator betting game
buy zithromax 500mg online – https://azithromycin.pro/can-i-take-zithromax-and-doxycycline-at-the-same-time.html zithromax 500mg price in india
aviator oyunu: aviator bahis – aviator sinyal hilesi
http://aviatormocambique.site/# aviator online
jogo de aposta: depósito mínimo 1 real – aviator jogo de aposta
how to get zithromax: is azithromycin the same as zithromax zithromax drug
aviator ghana: aviator game online – play aviator
aviator game online: aviator betting game – aviator game online
http://aviatormocambique.site/# aviator
where can i get zithromax: zithromax 500 mg lowest price pharmacy online – buy zithromax online with mastercard
pin up cassino online: pin-up cassino – aviator oficial pin up
zithromax 250mg – https://azithromycin.pro/over-the-counter-zithromax.html buy zithromax without presc
legal canadian pharmacy online: Cheapest drug prices Canada – canadian mail order pharmacy canadianpharm.store
buying prescription drugs in mexico online: Mexico pharmacy price list – п»їbest mexican online pharmacies mexicanpharm.shop
mexican rx online Medicines Mexico medicine in mexico pharmacies mexicanpharm.shop
https://mexicanpharm24.shop/# best online pharmacies in mexico mexicanpharm.shop
indianpharmacy com: Online India pharmacy – top online pharmacy india indianpharm.store
best india pharmacy india pharmacy mail order indian pharmacy online indianpharm.store
http://mexicanpharm24.shop/# reputable mexican pharmacies online mexicanpharm.shop
reputable indian online pharmacy: Pharmacies in India that ship to USA – online shopping pharmacy india indianpharm.store
buying prescription drugs in mexico online: Medicines Mexico – mexican border pharmacies shipping to usa mexicanpharm.shop
http://indianpharm24.shop/# world pharmacy india indianpharm.store
http://indianpharm24.shop/# indian pharmacy online indianpharm.store
https://canadianpharmlk.com/# canadian pharmacy king canadianpharm.store
https://mexicanpharm24.com/# mexico pharmacy mexicanpharm.shop
buy prescription drugs from india: buy prescription drugs from india – india pharmacy indianpharm.store
mexico drug stores pharmacies Medicines Mexico mexican rx online mexicanpharm.shop
http://canadianpharmlk.shop/# canadian pharmacy ed medications canadianpharm.store
http://mexicanpharm24.com/# mexico drug stores pharmacies mexicanpharm.shop
purple pharmacy mexico price list: order online from a Mexican pharmacy – medication from mexico pharmacy mexicanpharm.shop
http://indianpharm24.shop/# mail order pharmacy india indianpharm.store
certified canadian pharmacy: Cheapest drug prices Canada – canadian pharmacy no scripts canadianpharm.store
http://canadianpharmlk.shop/# canadianpharmacyworld canadianpharm.store
http://mexicanpharm24.shop/# mexico pharmacy mexicanpharm.shop
https://mexicanpharm24.shop/# best online pharmacies in mexico mexicanpharm.shop
indian pharmacy: Pharmacies in India that ship to USA – Online medicine home delivery indianpharm.store
http://canadianpharmlk.com/# canadian pharmacy 24 canadianpharm.store
http://canadianpharmlk.shop/# canadian discount pharmacy canadianpharm.store
http://canadianpharmlk.com/# canadian drugstore online canadianpharm.store
medicine in mexico pharmacies Mexico pharmacy online reputable mexican pharmacies online mexicanpharm.shop
http://canadianpharmlk.shop/# canadian pharmacy phone number canadianpharm.store
mexican drugstore online: Mexico pharmacy online – mexican pharmacy mexicanpharm.shop
http://canadianpharmlk.shop/# reliable canadian online pharmacy canadianpharm.store
http://mexicanpharm24.com/# pharmacies in mexico that ship to usa mexicanpharm.shop
mexican online pharmacies prescription drugs: mexican pharmacy – reputable mexican pharmacies online mexicanpharm.shop
https://indianpharm24.shop/# reputable indian online pharmacy indianpharm.store
https://mexicanpharm24.shop/# mexican border pharmacies shipping to usa mexicanpharm.shop
п»їbest mexican online pharmacies: order online from a Mexican pharmacy – mexico drug stores pharmacies mexicanpharm.shop
http://canadianpharmlk.com/# canadian online drugs canadianpharm.store
http://indianpharm24.com/# india pharmacy mail order indianpharm.store
http://prednisonest.pro/# prednisone cream over the counter
can you get cheap clomid: signs clomid is working – can i order cheap clomid no prescription
amoxicillin tablet 500mg: buy amoxicillin – buy amoxicillin 500mg
prednisone 40 mg rx prednisone no rx prednisone 4mg tab
amoxicillin 500mg without prescription: buy cheap amoxicillin online – amoxicillin 500mg buy online canada
how to buy clomid without a prescription: cost of clomid without dr prescription – order clomid without a prescription
https://clomidst.pro/# how can i get cheap clomid without dr prescription
amoxicillin online canada: how to get amoxicillin over the counter – amoxicillin online purchase
prednisone 20mg cheap: prednisone 2.5 mg daily – prednisone pack
amoxicillin tablet 500mg: can i drink alcohol while taking amoxicillin – azithromycin amoxicillin
https://prednisonest.pro/# prednisone in canada
how to get generic clomid pill: clomid women – can you get cheap clomid prices
can i buy prednisone from canada without a script generic prednisone pills 3000mg prednisone
where buy generic clomid tablets: where to buy cheap clomid online – can i get generic clomid without insurance
https://prednisonest.pro/# prednisone online paypal
where can i get prednisone: ibuprofen and prednisone – 5 prednisone in mexico
where to buy cheap clomid without insurance: can you get clomid for sale – buying generic clomid price
how to get clomid pills: where to get clomid no prescription – cost cheap clomid without prescription
prednisone 10 mg tablet: prednisone 2.5 mg price – prednisone 20mg capsule
http://clomidst.pro/# clomid order
clomid: where to buy clomid – buy clomid no prescription
clomid rx: п»їclomid in men – where to get cheap clomid prices
where can i buy amoxicillin online: medicine amoxicillin 500mg – buy amoxicillin 500mg online
prednisone over the counter cost prednisone pharmacy prices prednisone buy
https://amoxilst.pro/# where to buy amoxicillin pharmacy
buying clomid for sale: clomid coupon – where can i buy generic clomid without prescription
where can i buy cheap clomid without a prescription: clomid dose for men – can i purchase generic clomid without prescription
order amoxicillin online uk: can you buy amoxicillin over the counter in canada – canadian pharmacy amoxicillin
where buy generic clomid without rx: buying clomid prices – where to get generic clomid pills
http://amoxilst.pro/# amoxicillin 500 mg brand name
buy prescription drugs online without: discount prescription drugs canada – buying prescription drugs from canada online
https://onlinepharmacy.cheap/# online pharmacy without prescription
best online pharmacy without prescriptions: canadian prescriptions in usa – cheap prescription medication online
http://onlinepharmacy.cheap/# canadian pharmacy coupon code
cheapest ed meds: erectile dysfunction medication online – where can i buy ed pills
online pharmacy canada no prescription: non prescription online pharmacy india – no prescription drugs
cheapest online ed treatment: ed meds on line – cost of ed meds
where can i get ed pills buy ed pills online cheap ed meds online
http://onlinepharmacy.cheap/# rx pharmacy no prescription
no prescription required pharmacy: best online pharmacy – no prescription required pharmacy
http://edpills.guru/# online erectile dysfunction medication
best ed meds online: ed medications online – where to buy ed pills
http://pharmnoprescription.pro/# online prescription canada
online pharmacy without prescription: online pharmacy india – canadian pharmacy no prescription
canadian online pharmacy no prescription: mexican online pharmacy – cheapest pharmacy for prescriptions
order ed pills: ed treatment online – online ed meds
http://onlinepharmacy.cheap/# prescription free canadian pharmacy
http://pharmnoprescription.pro/# no prescription drugs
meds online without prescription: canadian mail order prescriptions – buying prescription medications online
offshore pharmacy no prescription: canadian online pharmacy – offshore pharmacy no prescription
cheap ed treatment order ed meds online pills for ed online
discount ed meds: cheap ed pills – online ed treatments
http://onlinepharmacy.cheap/# online pharmacy discount code
ed rx online: where to buy ed pills – cheap ed pills
non prescription online pharmacy: buying drugs without prescription – no prescription needed
https://pharmnoprescription.pro/# meds no prescription
https://pharmnoprescription.pro/# canadian prescription prices
online pharmacy prescription: mexican pharmacy online – prescription drugs from canada
mexican drugstore online: mexico pharmacy – buying from online mexican pharmacy
buying prescription medications online buy prescription drugs online without no prescription medicine
http://canadianpharm.guru/# my canadian pharmacy
buy medicines online in india: buy medicines online in india – india online pharmacy
medicine in mexico pharmacies: purple pharmacy mexico price list – п»їbest mexican online pharmacies
http://canadianpharm.guru/# canadian drug stores
https://canadianpharm.guru/# legitimate canadian pharmacy
online canadian drugstore: legitimate canadian pharmacy – my canadian pharmacy review
canada ed drugs: pharmacy rx world canada – canadian pharmacy online ship to usa
medicine in mexico pharmacies mexican border pharmacies shipping to usa п»їbest mexican online pharmacies
http://canadianpharm.guru/# canadian online drugstore
buy drugs from canada: prescription drugs canada buy online – canadian world pharmacy
https://indianpharm.shop/# pharmacy website india
medications online without prescription: online pharmacies no prescription usa – quality prescription drugs canada
best no prescription online pharmacies: online pharmacy not requiring prescription – prescription drugs online canada
https://indianpharm.shop/# indian pharmacies safe
purple pharmacy mexico price list: buying prescription drugs in mexico – mexican rx online
buy prescription drugs online without: prescription online canada – best non prescription online pharmacy
indian pharmacy paypal: indian pharmacy – best online pharmacy india
http://mexicanpharm.online/# mexican online pharmacies prescription drugs
mexican online pharmacies prescription drugs: buying from online mexican pharmacy – buying prescription drugs in mexico
mail order pharmacy india top online pharmacy india indian pharmacy
buying drugs without prescription: order prescription from canada – pharmacies without prescriptions
online shopping pharmacy india: Online medicine order – indian pharmacy
http://indianpharm.shop/# indian pharmacy
purple pharmacy mexico price list: reputable mexican pharmacies online – purple pharmacy mexico price list
https://canadianpharm.guru/# onlinecanadianpharmacy 24
india pharmacy mail order: online pharmacy india – world pharmacy india
top 10 online pharmacy in india: indian pharmacy paypal – indian pharmacy online
http://canadianpharm.guru/# canadian pharmacy prices
mexican mail order pharmacies: pharmacies in mexico that ship to usa – reputable mexican pharmacies online
buy pills without prescription: prescription from canada – canadian and international prescription service
canada pharmacy online: pharmacy canadian – canada discount pharmacy
https://mexicanpharm.online/# mexican drugstore online
buy medication online no prescription canada prescription online meds without prescription
online shopping pharmacy india: legitimate online pharmacies india – indian pharmacy
best canadian online pharmacy: certified canadian international pharmacy – www canadianonlinepharmacy
https://pharmacynoprescription.pro/# buying prescription medicine online
canada ed drugs: canadian neighbor pharmacy – prescription drugs canada buy online
reputable indian pharmacies: buy prescription drugs from india – top 10 online pharmacy in india
http://canadianpharm.guru/# 77 canadian pharmacy
online pharmacy canada no prescription: buying prescription medicine online – no prescription canadian pharmacies
top online pharmacy india: indian pharmacy – reputable indian online pharmacy
https://pharmacynoprescription.pro/# prescription canada
my canadian pharmacy review: canadapharmacyonline – best canadian pharmacy
online pharmacies no prescription: online pharmacy without a prescription – buy pills without prescription
reputable mexican pharmacies online: reputable mexican pharmacies online – buying prescription drugs in mexico online
pharmacy canadian superstore: canadianpharmacymeds – canadian mail order pharmacy
canadian drugstore online escrow pharmacy canada best canadian pharmacy online
http://indianpharm.shop/# india pharmacy mail order
https://pharmacynoprescription.pro/# no prescription medication
medicine in mexico pharmacies: purple pharmacy mexico price list – pharmacies in mexico that ship to usa
mexico pharmacies prescription drugs: reputable mexican pharmacies online – mexican rx online
http://indianpharm.shop/# online pharmacy india
mexican online pharmacies prescription drugs: buying prescription drugs in mexico online – mexico drug stores pharmacies
global pharmacy canada: canadian pharmacy world – certified canadian international pharmacy
mexican pharmaceuticals online: medication from mexico pharmacy – mexican mail order pharmacies
http://canadianpharm.guru/# best online canadian pharmacy
best rated canadian pharmacy: canadian pharmacy com – thecanadianpharmacy
purchasing prescription drugs online: online pharmacy without prescription – online pharmacy with prescription
buy drugs from canada vipps approved canadian online pharmacy canadian drug
online shopping pharmacy india: indian pharmacy online – india online pharmacy
https://pharmacynoprescription.pro/# how to buy prescriptions from canada safely
world pharmacy india: india pharmacy – buy prescription drugs from india
https://pharmacynoprescription.pro/# how to order prescription drugs from canada
mexican pharmacies no prescription: buy drugs online no prescription – online medicine without prescription
online pharmacy india: indian pharmacy online – buy medicines online in india
http://mexicanpharm.online/# mexico drug stores pharmacies
non prescription pharmacy: online pharmacy no prescription needed – online pharmacy canada no prescription
safe canadian pharmacy: my canadian pharmacy review – pharmacy in canada
п»їlegitimate online pharmacies india: п»їlegitimate online pharmacies india – india pharmacy mail order
cheap canadian pharmacy: canadian drug pharmacy – canadian pharmacy in canada
pin up casino guncel giris: pin-up casino indir – pin up casino giris
bonus veren casino slot siteleri: oyun siteleri slot – slot oyunlar? siteleri
https://gatesofolympus.auction/# gates of olympus oyna demo
http://slotsiteleri.guru/# bonus veren casino slot siteleri
https://aviatoroyna.bid/# aviator oyunu
pin up giris: aviator pin up – pin up
http://pinupgiris.fun/# pin up casino indir
https://pinupgiris.fun/# aviator pin up
deneme bonusu veren slot siteleri: slot siteleri bonus veren – deneme bonusu veren siteler
https://gatesofolympus.auction/# gates of olympus demo free spin
slot oyunlari: pragmatic play sweet bonanza – sweet bonanza hilesi
http://sweetbonanza.bid/# pragmatic play sweet bonanza
sweet bonanza kazanc: sweet bonanza siteleri – sweet bonanza indir
http://sweetbonanza.bid/# sweet bonanza demo oyna
pin up bet: pin up guncel giris – pin-up giris
https://gatesofolympus.auction/# gates of olympus slot
https://aviatoroyna.bid/# aviator bahis
gates of olympus hilesi: gates of olympus hilesi – pragmatic play gates of olympus
https://slotsiteleri.guru/# slot bahis siteleri
slot kumar siteleri: slot siteleri guvenilir – slot siteleri guvenilir
https://sweetbonanza.bid/# sweet bonanza 100 tl
slot siteleri bonus veren: guvenilir slot siteleri 2024 – bonus veren slot siteleri
http://sweetbonanza.bid/# sweet bonanza yorumlar
https://gatesofolympus.auction/# gates of olympus taktik
https://aviatoroyna.bid/# aviator oyunu 10 tl
aviator: aviator oyunu 20 tl – aviator mostbet
http://pinupgiris.fun/# pin-up online
ucak oyunu bahis aviator: aviator oyunu giris – aviator oyunu 100 tl
ucak oyunu bahis aviator: aviator bahis – aviator oyunu giris
http://gatesofolympus.auction/# gates of olympus demo türkçe oyna
gates of olympus slot: gates of olympus nas?l para kazanilir – gates of olympus oyna demo
http://gatesofolympus.auction/# gates of olympus demo
https://slotsiteleri.guru/# en güvenilir slot siteleri
gates of olympus hilesi: gates of olympus demo turkce oyna – gates of olympus hilesi
https://slotsiteleri.guru/# slot kumar siteleri
aviator oyunu 10 tl: aviator bahis – aviator oyna slot
http://sweetbonanza.bid/# sweet bonanza kazanç
mexico drug stores pharmacies best online pharmacies in mexico buying prescription drugs in mexico
https://indianpharmacy.icu/# india pharmacy
medication from mexico pharmacy: cheapest mexico drugs – buying prescription drugs in mexico online
canadian online pharmacy reviews pills now even cheaper canadian pharmacy ltd
online shopping pharmacy india: indian pharmacy delivery – indian pharmacy online
onlinecanadianpharmacy 24 canadian pharmacy 24 certified canadian pharmacy
best india pharmacy: indian pharmacy – top online pharmacy india
https://mexicanpharmacy.shop/# mexico pharmacies prescription drugs
mexico drug stores pharmacies cheapest mexico drugs п»їbest mexican online pharmacies
indian pharmacy paypal: Healthcare and medicines from India – india pharmacy mail order
cheapest online pharmacy india: online shopping pharmacy india – reputable indian online pharmacy
mexico drug stores pharmacies: mexico pharmacy – reputable mexican pharmacies online
indianpharmacy com: Healthcare and medicines from India – indian pharmacy online
is canadian pharmacy legit best online canadian pharmacy canadian drugs pharmacy
india pharmacy mail order: Healthcare and medicines from India – indian pharmacies safe
best canadian online pharmacy: Licensed Canadian Pharmacy – best canadian pharmacy
online pharmacy india: best online pharmacy india – Online medicine home delivery
https://indianpharmacy.icu/# п»їlegitimate online pharmacies india
drugs from canada Large Selection of Medications global pharmacy canada
cheapest online pharmacy india: india pharmacy – indianpharmacy com
pharmacy com canada: Prescription Drugs from Canada – online pharmacy canada
best canadian pharmacy online: Prescription Drugs from Canada – vipps canadian pharmacy
indian pharmacy paypal Generic Medicine India to USA indian pharmacy
mexico pharmacies prescription drugs: mexico drug stores pharmacies – mexican pharmaceuticals online
mexican online pharmacies prescription drugs: Online Pharmacies in Mexico – medicine in mexico pharmacies
best india pharmacy: Generic Medicine India to USA – buy prescription drugs from india
mexico drug stores pharmacies: Mexican Pharmacy Online – pharmacies in mexico that ship to usa
top 10 pharmacies in india Cheapest online pharmacy top online pharmacy india
medication from mexico pharmacy: Online Pharmacies in Mexico – mexican border pharmacies shipping to usa
http://indianpharmacy.icu/# india online pharmacy
canada cloud pharmacy pills now even cheaper canadian drug prices
safe canadian pharmacies: Certified Canadian Pharmacy – canadian pharmacy no scripts
recommended canadian pharmacies: pills now even cheaper – legitimate canadian pharmacy online
indian pharmacy paypal Cheapest online pharmacy Online medicine order
reputable indian pharmacies: indian pharmacy – buy prescription drugs from india
world pharmacy india: Generic Medicine India to USA – indian pharmacies safe
indian pharmacy Generic Medicine India to USA mail order pharmacy india
medicine in mexico pharmacies: Mexican Pharmacy Online – mexican mail order pharmacies
https://canadianpharmacy24.store/# my canadian pharmacy rx
non prescription prednisone 20mg 20 mg prednisone prednisone 50 mg for sale
https://amoxilall.shop/# amoxicillin where to get
amoxicillin price without insurance: amoxicillin brand name – where can you buy amoxicillin over the counter
http://clomidall.shop/# clomid online
https://amoxilall.shop/# amoxicillin generic
zithromax price south africa zithromax 500 tablet zithromax online
http://clomidall.shop/# how to get generic clomid tablets
http://zithromaxall.shop/# buy zithromax canada
zithromax 500mg zithromax z-pak can i buy zithromax online
prednisone 40 mg price: 50mg prednisone tablet – generic prednisone 10mg
http://prednisoneall.shop/# online prednisone 5mg
generic prednisone for sale purchase prednisone canada prednisone 10 mg canada
https://amoxilall.shop/# amoxicillin 500 mg tablet price
zithromax tablets for sale: where can i purchase zithromax online – generic zithromax azithromycin
http://amoxilall.shop/# amoxicillin 875 125 mg tab
amoxicillin buy canada amoxicillin 500 amoxicillin 1000 mg capsule
https://prednisoneall.com/# buy prednisone online usa
https://amoxilall.shop/# amoxicillin where to get
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
buy cheap generic zithromax buy zithromax 1000mg online where can i buy zithromax in canada
https://clomidall.com/# cost of clomid for sale
can you get clomid: can you buy clomid without prescription – where can i buy cheap clomid without prescription
https://clomidall.com/# where to buy clomid without dr prescription
can i get clomid without rx where can i get generic clomid no prescription can i buy cheap clomid
https://clomidall.shop/# can you get clomid
how to buy zithromax online: buy cheap zithromax online – where can i buy zithromax capsules
https://prednisoneall.shop/# prednisone 15 mg tablet
http://zithromaxall.shop/# zithromax antibiotic
amoxicillin pills 500 mg where can you buy amoxicillin over the counter buying amoxicillin in mexico
https://clomidall.com/# get cheap clomid price
https://clomidall.com/# can you buy cheap clomid without dr prescription
prednisone buy no prescription prednisone 300mg prednisone 50 mg coupon
http://prednisoneall.com/# 20 mg prednisone
prednisone otc price: prednisone 30 mg tablet – canada pharmacy prednisone
cheapest cialis Tadalafil Tablet Cialis 20mg price in USA
generic sildenafil: buy viagra online – viagra canada
https://tadalafiliq.shop/# Cialis 20mg price in USA
Cialis 20mg price in USA cialis best price Generic Tadalafil 20mg price
cheap kamagra: Sildenafil Oral Jelly – cheap kamagra
https://sildenafiliq.com/# cheap viagra
Kamagra tablets: Kamagra gel – Kamagra 100mg price
buy Viagra over the counter generic ed pills cheapest viagra
buy viagra here: Cheap generic Viagra – viagra without prescription
https://kamagraiq.shop/# Kamagra 100mg price
cialis generic: Generic Tadalafil 20mg price – cheapest cialis
Buy Tadalafil 20mg Buy Cialis online buy cialis pill
Cheap generic Viagra online: generic ed pills – sildenafil online
https://kamagraiq.com/# Kamagra 100mg price
sildenafil oral jelly 100mg kamagra: Kamagra tablets – Kamagra tablets
https://sildenafiliq.xyz/# Viagra online price
best price for viagra 100mg buy viagra online Order Viagra 50 mg online
Buy Tadalafil 5mg: tadalafil iq – cialis for sale
Sildenafil Citrate Tablets 100mg: cheapest viagra – Sildenafil 100mg price
Kamagra 100mg price: Kamagra gel – Kamagra tablets
https://sildenafiliq.xyz/# generic sildenafil
Kamagra 100mg price Sildenafil Oral Jelly super kamagra
http://kamagraiq.com/# super kamagra
Buy Tadalafil 10mg: tadalafil iq – Tadalafil price
Viagra without a doctor prescription Canada generic ed pills generic sildenafil
https://kamagraiq.shop/# Kamagra tablets
buy cialis pill: cheapest cialis – Cialis 20mg price
https://sildenafiliq.com/# Buy Viagra online cheap
Kamagra 100mg price: buy Kamagra – Kamagra 100mg price
https://tadalafiliq.com/# Tadalafil Tablet
buy kamagra online usa kamagra best price п»їkamagra
kamagra: Kamagra Oral Jelly Price – Kamagra 100mg price
https://sildenafiliq.xyz/# Viagra generic over the counter
Cheap Viagra 100mg: best price on viagra – Viagra online price
Generic Viagra for sale: generic ed pills – Viagra without a doctor prescription Canada
Cialis over the counter Buy Cialis online Buy Tadalafil 10mg
https://sildenafiliq.xyz/# Viagra Tablet price
Generic Tadalafil 20mg price: cialis best price – Generic Tadalafil 20mg price
http://kamagraiq.shop/# Kamagra 100mg price
https://kamagraiq.com/# Kamagra 100mg
sildenafil oral jelly 100mg kamagra Kamagra Oral Jelly Price buy Kamagra
best price for viagra 100mg: cheapest viagra – sildenafil 50 mg price
https://tadalafiliq.com/# Cialis without a doctor prescription
cheapest cialis: Buy Cialis online – Tadalafil price
https://sildenafiliq.com/# buy Viagra over the counter
Cialis 20mg price in USA Buy Cialis online Buy Tadalafil 5mg
https://kamagraiq.com/# buy Kamagra
buy viagra here: generic ed pills – Viagra generic over the counter
mexican rx online mexican pharmacy mexico drug stores pharmacies
http://mexicanpharmgrx.com/# mexican pharmaceuticals online
canadian pharmacy online reviews International Pharmacy delivery canadian pharmacies compare
canadian mail order pharmacy: International Pharmacy delivery – buy prescription drugs from canada cheap
mexican rx online: online pharmacy in Mexico – best online pharmacies in mexico
https://mexicanpharmgrx.shop/# mexican online pharmacies prescription drugs
best india pharmacy Healthcare and medicines from India Online medicine order
https://indianpharmgrx.shop/# indian pharmacy online
top 10 pharmacies in india: indian pharmacy delivery – india online pharmacy
https://canadianpharmgrx.com/# legal canadian pharmacy online
top 10 online pharmacy in india indian pharmacy top 10 pharmacies in india
best rated canadian pharmacy: Canada pharmacy online – best canadian pharmacy
https://mexicanpharmgrx.com/# buying from online mexican pharmacy
mail order pharmacy india indianpharmacy com п»їlegitimate online pharmacies india
http://indianpharmgrx.shop/# indianpharmacy com
best online canadian pharmacy: Canada pharmacy online – pharmacy wholesalers canada
top 10 online pharmacy in india: indian pharmacy delivery – buy prescription drugs from india
canadian drugs pharmacy International Pharmacy delivery canadian online pharmacy reviews
http://indianpharmgrx.shop/# world pharmacy india
mexican drugstore online: buying prescription drugs in mexico online – п»їbest mexican online pharmacies
https://mexicanpharmgrx.com/# mexican rx online
protonix classification
buying prescription drugs in mexico mexican pharmacy buying from online mexican pharmacy
http://indianpharmgrx.shop/# world pharmacy india
ed drugs online from canada: Cheapest drug prices Canada – legitimate canadian pharmacies
indian pharmacies safe indian pharmacy india online pharmacy
generic protonix
http://indianpharmgrx.com/# top 10 online pharmacy in india
mexico drug stores pharmacies: reputable mexican pharmacies online – buying prescription drugs in mexico online
http://canadianpharmgrx.xyz/# canadian pharmacy 365
buying prescription drugs in mexico online buying prescription drugs in mexico online buying prescription drugs in mexico online
canadapharmacyonline com: canadian pharmacy online store – canadian pharmacy prices
http://indianpharmgrx.shop/# pharmacy website india
medication from mexico pharmacy: mexican pharmacy – medicine in mexico pharmacies
https://canadianpharmgrx.xyz/# reliable canadian pharmacy
canadian pharmacy meds Best Canadian online pharmacy best rated canadian pharmacy
http://indianpharmgrx.shop/# india online pharmacy
mail order pharmacy india: Healthcare and medicines from India – india pharmacy
india online pharmacy: indian pharmacy – india pharmacy mail order
doxycycline generic buy doxycycline doxycycline generic
https://nolvadex.icu/# buy nolvadex online
buy cytotec: Abortion pills online – Misoprostol 200 mg buy online
diflucan 150 mg fluconazole diflucan 150 cost can you purchase diflucan
how to buy diflucan: how to get diflucan online – where can i buy diflucan without a prescription
generic for doxycycline: order doxycycline 100mg without prescription – doxycycline 50 mg
buy diflucan pill: diflucan generic brand – how can i get diflucan over the counter
tamoxifen depression who should take tamoxifen generic tamoxifen
medication diflucan price: diflucan.com – purchase diflucan online
ciprofloxacin generic price: buy cipro – ciprofloxacin 500 mg tablet price
cytotec online: buy misoprostol over the counter – buy cytotec over the counter
doxycycline vibramycin doxycycline hyclate 100 mg cap odering doxycycline
tamoxifen depression: tamoxifen – nolvadex price
https://nolvadex.icu/# benefits of tamoxifen
ciprofloxacin over the counter: buy ciprofloxacin over the counter – ciprofloxacin over the counter
cytotec online buy cytotec online fast delivery cytotec buy online usa
Misoprostol 200 mg buy online: buy cytotec – buy cytotec pills online cheap
doxycycline 100mg dogs: doxycycline 50 mg – order doxycycline 100mg without prescription
doxycycline 100mg price: doxycycline hyclate – doxycycline monohydrate
tamoxifen skin changes natural alternatives to tamoxifen nolvadex d
where can i buy nolvadex: tamoxifen vs raloxifene – nolvadex d
where can i buy cipro online buy cipro online without prescription ciprofloxacin order online
purchase diflucan online: diflucan medicine in india – order diflucan
cheap doxycycline online: doxy 200 – buy doxycycline online 270 tabs
https://doxycyclinest.pro/# doxycycline prices
buy cipro cheap: buy ciprofloxacin tablets – buy cipro online
tamoxifen vs clomid: nolvadex for pct – what is tamoxifen used for
buy cipro cheap ciprofloxacin 500 mg tablet price п»їcipro generic
ciprofloxacin over the counter: cipro 500mg best prices – cipro ciprofloxacin
can you order diflucan online how to buy diflucan online where to get diflucan otc
buy cytotec in usa: buy cytotec in usa – purchase cytotec
buy diflucan canada: diflucan mexico – where to buy diflucan in singapore
buy diflucan online usa: buy online diflucan – diflucan 150 mg prescription
diflucan pills online: diflucan price south africa – diflucan 400mg without prescription
https://diflucan.icu/# where can i purchase diflucan
cost of diflucan: diflucan pills prescription – diflucan coupon canada
cipro п»їcipro generic ciprofloxacin
buy diflucan without prescription: diflucan tablets – diflucan price canada
generic diflucan: diflucan tablet 500mg – can i buy diflucan in mexico
cipro 500mg best prices: buy ciprofloxacin – ciprofloxacin over the counter
prednisone tabs 20 mg brand prednisone prednisone price south africa
can you buy clomid without prescription: can i buy cheap clomid tablets – can i get clomid price
prednisone in india: prednisone without prescription – prednisone pill 10 mg
buy zithromax online fast shipping zithromax over the counter uk where can i buy zithromax uk
ivermectin rx: buy stromectol uk – stromectol tablets buy online
stromectol 3 mg dosage: stromectol coronavirus – stromectol tablets uk
http://amoxicillina.top/# where can i buy amoxicillin over the counter
http://prednisonea.store/# cost of prednisone in canada
stromectol otc stromectol uk buy ivermectin usa
ivermectin where to buy: stromectol 15 mg – ivermectin human
can you buy zithromax over the counter in australia: cost of generic zithromax – zithromax antibiotic
zithromax purchase online: zithromax 500 price – zithromax generic cost
https://amoxicillina.top/# amoxicillin canada price
zithromax cost uk can you buy zithromax over the counter in mexico zithromax over the counter uk
ivermectin uk: stromectol tablets 3 mg – ivermectin nz
6 prednisone: prednisone 20mg capsule – prednisone without prescription
https://stromectola.top/# how to buy stromectol
how to get prednisone without a prescription: prednisone 50 mg buy – prednisone 30
where can i get cheap clomid no prescription where can i buy clomid no prescription cost generic clomid for sale
https://clomida.pro/# can i order clomid for sale
clomid generics: cheap clomid without a prescription – where can i buy clomid without prescription
https://stromectola.top/# ivermectin 3 mg dose
cheap amoxicillin 500mg: order amoxicillin uk – can you purchase amoxicillin online
80 mg prednisone daily prednisone 2.5 mg price prednisone 5443
cost of generic zithromax: buy zithromax online cheap – can i buy zithromax online
canadian and international prescription service: canada online prescription – order prescription drugs online without doctor
online pharmacy no prescription needed: rxpharmacycoupons – online pharmacy without prescription
https://medicationnoprescription.pro/# online drugs no prescription
http://onlinepharmacyworld.shop/# prescription drugs online
no prescription needed pharmacy canadian pharmacy without prescription canadian pharmacy coupon
cheapest ed medication: buying erectile dysfunction pills online – erectile dysfunction pills for sale
https://medicationnoprescription.pro/# online pharmacy no prescription
https://edpill.top/# buy erectile dysfunction treatment
ed medicine online cost of ed meds online ed prescription
ed prescription online: ed rx online – low cost ed pills
https://edpill.top/# ed medicines
http://medicationnoprescription.pro/# online drugstore no prescription
https://medicationnoprescription.pro/# buying prescription medications online
canadian pharmacy no prescription: no prescription canadian pharmacy – buying prescription drugs online without a prescription
no prescription medication cheap prescription drugs online no prescription medicine
cheap erection pills: ed pills – pills for erectile dysfunction online
https://edpill.top/# online ed medication
canadian pharmacy discount code: canada pharmacy not requiring prescription – cheapest pharmacy for prescriptions
https://onlinepharmacyworld.shop/# canadian pharmacies not requiring prescription
https://medicationnoprescription.pro/# п»їonline pharmacy no prescription needed
http://edpill.top/# where to buy ed pills
canadian prescriptions in usa canada prescription drugs online medicine with no prescription
https://edpill.top/# best ed pills online
canada online pharmacy no prescription: offshore pharmacy no prescription – online pharmacy prescription
casino online uy tin web c? b?c online uy tin casino tr?c tuy?n vi?t nam
casino tr?c tuy?n: casino tr?c tuy?n vi?t nam – casino online uy tín
casino online uy tin casino online uy tin casino tr?c tuy?n uy tin
https://casinvietnam.com/# casino tr?c tuy?n
danh bai tr?c tuy?n casino tr?c tuy?n uy tin casino tr?c tuy?n
Step 1First, go to Google Contacts. Click on the More located on the top of Contacts-> Export -> Select contacts” and export them as Google CSV. Now, open the Contacts app, and you will see your Google Contacts. 5. You’ll see sliders for various Google services. Enable the slider to allow access to your Google contacts. SyncGene is online synchronization tool. Synchronize and manage your calendars, contacts and tasks across multiple online sources such as Google, iCloud and Exchange. Contacts are considered a significant part of the phone software. Many people do rely on their contacts for day-to-day communications. As such, they try all means to keep them safe. Keeping your contacts in Google is the safest way to keep them intact. And when you want to import Google contacts to the iPhone, it will be very easy.
https://eworldui.azurewebsites.net/blog/post/2005/11/12/Its-Finally-Live!-Check-out-the-New-Partner-Membership-Center-Website.aspx
Open your Gmail, and import the .csv file to your account. First, save all contacts on the Android phone to its SIM. Next, insert the SIM into your iPhone, taking care not to mislay the iPhone’s SIM. Finally, go to Settings and select Contacts (or Mail, Contacts, Calendars in older versions of iOS) and tap Import SIM Contacts. iOS has native contact syncing for cloud based accounts. For instance, you can connect multiple accounts like iCloud, Gmail, and Exchange and display all the contacts stored in those accounts. You can also select a default account so any new contact you save won’t be duplicated across all linked accounts. Make sure your Google Account is connected to your iPhone or iPad using SSL, the recommended secure connection. Go to the Phone app and the Contact section and you should see contacts start to appear in there from Google Contacts.
choi casino tr?c tuy?n tren di?n tho?i casino tr?c tuy?n choi casino tr?c tuy?n tren di?n tho?i
game c? b?c online uy tín: casino tr?c tuy?n uy tín – casino online uy tín
https://casinvietnam.shop/# danh bai tr?c tuy?n
casino tr?c tuy?n vi?t nam: casino tr?c tuy?n – game c? b?c online uy tín
casino tr?c tuy?n: casino tr?c tuy?n vi?t nam – casino tr?c tuy?n vi?t nam
game c? b?c online uy tín: casino online uy tín – dánh bài tr?c tuy?n
casino tr?c tuy?n vi?t nam: casino tr?c tuy?n vi?t nam – choi casino tr?c tuy?n trên di?n tho?i
dánh bài tr?c tuy?n: dánh bài tr?c tuy?n – dánh bài tr?c tuy?n
casino tr?c tuy?n vi?t nam: casino tr?c tuy?n vi?t nam – web c? b?c online uy tín
web c? b?c online uy tin casino tr?c tuy?n vi?t nam game c? b?c online uy tin
casino tr?c tuy?n uy tín: casino tr?c tuy?n uy tín – casino online uy tín
game c? b?c online uy tin choi casino tr?c tuy?n tren di?n tho?i casino tr?c tuy?n vi?t nam
casino tr?c tuy?n vi?t nam choi casino tr?c tuy?n tren di?n tho?i game c? b?c online uy tin
casino tr?c tuy?n: choi casino tr?c tuy?n trên di?n tho?i – casino tr?c tuy?n vi?t nam
casino tr?c tuy?n: web c? b?c online uy tín – casino tr?c tuy?n uy tín
web c? b?c online uy tin casino online uy tin game c? b?c online uy tin
medicine in mexico pharmacies mexico pharmacy best online pharmacies in mexico
http://indiaph24.store/# pharmacy website india
mail order pharmacy india Cheapest online pharmacy buy medicines online in india
https://canadaph24.pro/# canadian pharmacy king reviews
indian pharmacies safe Generic Medicine India to USA top online pharmacy india
https://indiaph24.store/# india online pharmacy
cross border pharmacy canada Large Selection of Medications from Canada best canadian pharmacy to order from
http://mexicoph24.life/# mexican drugstore online
reputable indian online pharmacy indian pharmacy reputable indian pharmacies
http://mexicoph24.life/# mexican pharmaceuticals online
canadian online drugstore Certified Canadian Pharmacies best online canadian pharmacy
https://canadaph24.pro/# www canadianonlinepharmacy
cheapest online pharmacy india buy medicines online in india top 10 pharmacies in india
https://canadaph24.pro/# canadian family pharmacy
mexico pharmacies prescription drugs Mexican Pharmacy Online п»їbest mexican online pharmacies
http://canadaph24.pro/# canadian pharmacies comparison
canadian pharmacy no scripts Large Selection of Medications from Canada cheap canadian pharmacy
https://canadaph24.pro/# global pharmacy canada
buy medicines online in india Cheapest online pharmacy india pharmacy mail order
http://canadaph24.pro/# canadian pharmacy online
northwest pharmacy canada Large Selection of Medications from Canada safe canadian pharmacy
http://canadaph24.pro/# buying drugs from canada
canadian pharmacy cheap canadian pharmacies online canadian pharmacy
https://indiaph24.store/# india online pharmacy
canada drugs Licensed Canadian Pharmacy canadian online drugs
https://mexicoph24.life/# mexican pharmacy
adderall canadian pharmacy Certified Canadian Pharmacies canadian compounding pharmacy
https://canadaph24.pro/# canadian pharmacy online
legit canadian pharmacy buying from canadian pharmacies canadian pharmacy 365
https://canadaph24.pro/# canadian pharmacy 365
п»їbest mexican online pharmacies cheapest mexico drugs buying from online mexican pharmacy
https://mexicoph24.life/# pharmacies in mexico that ship to usa
mexican drugstore online Mexican Pharmacy Online medication from mexico pharmacy
http://mexicoph24.life/# mexico drug stores pharmacies
canadian drug stores Licensed Canadian Pharmacy canadian discount pharmacy
https://canadaph24.pro/# my canadian pharmacy reviews
mail order pharmacy india indian pharmacy fast delivery mail order pharmacy india
http://mexicoph24.life/# mexican mail order pharmacies
online shopping pharmacy india indian pharmacy fast delivery indian pharmacy online
canadian pharmacy oxycodone canadian pharmacies certified canadian pharmacy
https://mexicoph24.life/# mexico drug stores pharmacies
pharmacy website india Generic Medicine India to USA reputable indian online pharmacy
http://canadaph24.pro/# pharmacy in canada
canadian pharmacy in canada Prescription Drugs from Canada canadian pharmacy ltd
http://canadaph24.pro/# canadian pharmacy price checker
https://indiaph24.store/# legitimate online pharmacies india
canadian valley pharmacy Large Selection of Medications from Canada canadian pharmacy scam
http://mexicoph24.life/# best mexican online pharmacies
mexican drugstore online mexico pharmacy best online pharmacies in mexico
http://indiaph24.store/# india pharmacy mail order
best india pharmacy Cheapest online pharmacy п»їlegitimate online pharmacies india
Online medicine order Cheapest online pharmacy india online pharmacy
https://indiaph24.store/# Online medicine home delivery
http://canadaph24.pro/# pharmacy canadian
indianpharmacy com pharmacy website india cheapest online pharmacy india
reputable indian online pharmacy https://indiaph24.store/# online shopping pharmacy india
indian pharmacies safe
pharmacy website india indian pharmacy reputable indian online pharmacy
https://mexicoph24.life/# mexican pharmacy
top online pharmacy india pharmacy website india indian pharmacy online
https://mexicoph24.life/# mexico drug stores pharmacies
mexican online pharmacies prescription drugs: mexico pharmacy – п»їbest mexican online pharmacies
pharmacies in mexico that ship to usa cheapest mexico drugs pharmacies in mexico that ship to usa
https://indiaph24.store/# mail order pharmacy india
online shopping pharmacy india buy medicines from India indian pharmacy paypal
mexico pharmacies prescription drugs Mexican Pharmacy Online mexico drug stores pharmacies
http://indiaph24.store/# top online pharmacy india
http://mexicoph24.life/# reputable mexican pharmacies online
canadian pharmacy tampa canadian 24 hour pharmacy best canadian online pharmacy reviews
http://mexicoph24.life/# mexican drugstore online
mexico drug stores pharmacies mexican pharmacy mexican pharmaceuticals online
https://mexicoph24.life/# buying prescription drugs in mexico
mail order pharmacy india buy medicines from India india pharmacy
https://canadaph24.pro/# online canadian pharmacy
online shopping pharmacy india Generic Medicine India to USA Online medicine order
https://mexicoph24.life/# mexican drugstore online
best canadian pharmacy to buy from Licensed Canadian Pharmacy canadianpharmacymeds com
https://indiaph24.store/# india pharmacy mail order
mexican mail order pharmacies Mexican Pharmacy Online mexican drugstore online
http://canadaph24.pro/# canadian pharmacy
buying prescription drugs in mexico: mexico pharmacy – mexican pharmaceuticals online
http://mexicoph24.life/# mexican online pharmacies prescription drugs
purple pharmacy mexico price list cheapest mexico drugs mexico drug stores pharmacies
reliable canadian pharmacy reviews canadian pharmacies www canadianonlinepharmacy
https://canadaph24.pro/# best canadian online pharmacy
top 10 pharmacies in india https://indiaph24.store/# Online medicine order
indian pharmacy
https://indiaph24.store/# reputable indian pharmacies
best online pharmacies in mexico: mexican pharmacy – pharmacies in mexico that ship to usa
https://indiaph24.store/# india pharmacy
mexican online pharmacies prescription drugs mexican pharmacy buying prescription drugs in mexico online
https://nolvadex.life/# femara vs tamoxifen
buy cytotec online fast delivery: buy cytotec over the counter – buy cytotec in usa
buy cytotec over the counter cytotec buy online usa buy misoprostol over the counter
prinivil online: lisinopril 20 mg for sale – lisinopril 5 mg medicine
https://cytotec.club/# buy cytotec pills online cheap
http://lisinopril.network/# lisinopril 5 mg tablet price in india
buy cipro buy ciprofloxacin over the counter buy cipro online
effexor and tamoxifen tamoxifen medication nolvadex only pct
http://cytotec.club/# buy cytotec
antibiotics cipro: ciprofloxacin over the counter – cipro 500mg best prices
lisinopril 10mg: zestril cost price – buy zestril online
http://ciprofloxacin.tech/# where can i buy cipro online
https://finasteride.store/# propecia price
tamoxifen hot flashes tamoxifen menopause tamoxifen bone pain
tamoxifen and antidepressants nolvadex gynecomastia tamoxifen generic
http://cytotec.club/# purchase cytotec
propecia without prescription: cost generic propecia price – cost of generic propecia without rx
tamoxifen cyp2d6: liquid tamoxifen – tamoxifen cancer
buy propecia prices order cheap propecia pills buying cheap propecia without prescription
https://lisinopril.network/# lisinopril 20mg daily
http://cytotec.club/# buy cytotec in usa
https://lisinopril.network/# lisinopril 104
lisinopril 15 mg tablets: lisinopril 40 mg india – lisinopril without an rx
https://nolvadex.life/# tamoxifen menopause
п»їcipro generic ciprofloxacin 500 mg tablet price ciprofloxacin mail online
tamoxifen breast cancer is nolvadex legal what happens when you stop taking tamoxifen
tamoxifen and bone density: how to prevent hair loss while on tamoxifen – tamoxifen warning
http://ciprofloxacin.tech/# ciprofloxacin generic price
http://nolvadex.life/# tamoxifen for breast cancer prevention
cipro 500mg best prices buy cipro online without prescription buy cipro online canada
buy ciprofloxacin п»їcipro generic ciprofloxacin order online
cytotec pills buy online: buy cytotec online fast delivery – purchase cytotec
tamoxifen vs raloxifene: does tamoxifen cause bone loss – what is tamoxifen used for
https://ciprofloxacin.tech/# cipro ciprofloxacin
http://nolvadex.life/# tamoxifen hip pain
https://cytotec.club/# Abortion pills online
buy propecia for sale buy generic propecia get propecia online
buy cytotec over the counter Abortion pills online buy cytotec
cheap propecia without a prescription: buying propecia price – generic propecia without rx
prinivil 5 mg: lisinopril pill 40 mg – cost of lisinopril 10 mg
http://cytotec.club/# buy cytotec over the counter
http://lisinopril.network/# lisinopril 40 mg generic
cost cheap propecia without prescription cheap propecia without a prescription cost generic propecia without insurance
ciprofloxacin generic price cipro for sale buy cipro cheap
http://finasteride.store/# propecia prices
nolvadex steroids: nolvadex for sale – tamoxifen for gynecomastia reviews
lisinopril 20 mg canada: lisinopril generic over the counter – lisinopril 2.5 pill
http://ciprofloxacin.tech/# buy cipro
zestril price in india lisinopril in india lisinopril 40 mg discount
cost generic propecia no prescription buying propecia without a prescription order cheap propecia pills
http://ciprofloxacin.tech/# buy cipro online
http://cytotec.club/# Misoprostol 200 mg buy online
tamoxifen buy: nolvadex for sale – tamoxifen hip pain
cytotec buy online usa: Cytotec 200mcg price – buy cytotec pills
purchase cytotec buy misoprostol over the counter buy cytotec in usa
tamoxifen tamoxifen rash tamoxifen and ovarian cancer
https://finasteride.store/# cost generic propecia without insurance
http://nolvadex.life/# tamoxifen dose
https://cytotec.club/# buy cytotec
ciprofloxacin: cipro generic – ciprofloxacin 500 mg tablet price
cipro generic: ciprofloxacin – cipro for sale
tamoxifen and bone density tamoxifen 20 mg tamoxifen for breast cancer prevention
get propecia pills buying generic propecia price buy propecia pills
http://ciprofloxacin.tech/# antibiotics cipro
http://cytotec.club/# buy cytotec
ciprofloxacin mail online: cipro generic – where can i buy cipro online
cipro for sale ciprofloxacin order online ciprofloxacin
cytotec buy online usa buy cytotec online cytotec online
http://nolvadex.life/# nolvadex for pct
buy ciprofloxacin: buy cipro online – buy cipro cheap
https://nolvadex.life/# nolvadex only pct
http://lisinopril.network/# lisinopril 2.5 mg price
https://finasteride.store/# generic propecia without prescription
arimidex vs tamoxifen bodybuilding tamoxifen effectiveness nolvadex for pct
can you buy lisinopril over the counter prinivil cost lisinopril 5 mg uk price
tamoxifen dosage: common side effects of tamoxifen – tamoxifen skin changes
tamoxifen postmenopausal: tamoxifen bone pain – tamoxifen for sale
https://ciprofloxacin.tech/# purchase cipro
https://kamagra.win/# Kamagra Oral Jelly
buy kamagra online usa: kamagra oral jelly – Kamagra Oral Jelly
Levitra 20 mg for sale Levitra generic best price Buy Vardenafil 20mg
Kamagra 100mg price kamagra.win Kamagra 100mg price
Cheap generic Viagra online: Cheapest place to buy Viagra – Cheap Sildenafil 100mg
http://cenforce.pro/# Buy Cenforce 100mg Online
http://viagras.online/# over the counter sildenafil
http://kamagra.win/# Kamagra Oral Jelly
buy cenforce: cenforce.pro – Purchase Cenforce Online
buy Kamagra kamagra oral jelly п»їkamagra
levitra medication online
buy kamagra online usa cheap kamagra buy kamagra online usa
https://kamagra.win/# Kamagra 100mg
https://cialist.pro/# Tadalafil price
http://kamagra.win/# п»їkamagra
kamagra: kamagra – kamagra
cenforce.pro: cheapest cenforce – Purchase Cenforce Online
Generic Levitra 20mg: Levitra 20mg price – Levitra online pharmacy
https://cenforce.pro/# Cenforce 100mg tablets for sale
Kamagra 100mg price kamagra.win super kamagra
cenforce.pro order cenforce cheapest cenforce
https://viagras.online/# buy Viagra online
Cenforce 150 mg online: cenforce for sale – cenforce for sale
kamagra: kamagra – sildenafil oral jelly 100mg kamagra
https://cenforce.pro/# Cenforce 100mg tablets for sale
Levitra online USA fast Levitra 20mg price Cheap Levitra online
cialis generic: buy cialis overseas – cialis generic
http://kamagra.win/# sildenafil oral jelly 100mg kamagra
http://viagras.online/# Cheap generic Viagra
cheapest viagra: Buy Viagra online cheap – Cheap generic Viagra online
Generic Cialis price: buy cialis online – Generic Tadalafil 20mg price
Viagra generic over the counter viagras.online buy Viagra online
п»їkamagra kamagra oral jelly buy Kamagra
https://viagras.online/# viagra without prescription
Levitra 10 mg best price: Levitra generic price – Cheap Levitra online
Generic Viagra for sale: viagras.online – Viagra tablet online
https://cenforce.pro/# cenforce.pro
sildenafil oral jelly 100mg kamagra: buy kamagra online – Kamagra 100mg price
http://viagras.online/# viagra canada
sildenafil 50 mg price sildenafil over the counter cheap viagra
п»їcialis generic buy cialis pill Cialis over the counter
https://kamagra.win/# sildenafil oral jelly 100mg kamagra
http://kamagra.win/# buy kamagra online usa
Levitra generic best price: Vardenafil online prescription – Buy Vardenafil 20mg online
https://kamagra.win/# super kamagra
buy Viagra over the counter: Buy Viagra online – Cheap Sildenafil 100mg
buy Viagra over the counter buy Viagra over the counter Viagra generic over the counter
cheap viagra Buy Viagra online cheap cheap viagra
http://pharmindia.online/# indianpharmacy com
https://pharmworld.store/# offshore pharmacy no prescription
no prescription needed pharmacy: pharmacy coupons – buying prescription drugs from canada
quality prescription drugs canada online pharmacy canada no prescription buy medications online no prescription
online pharmacy without a prescription pharmacy with no prescription canadian pharmacy no prescription required
mail order pharmacy india: world pharmacy india – top 10 online pharmacy in india
http://pharmindia.online/# п»їlegitimate online pharmacies india
http://pharmcanada.shop/# canada pharmacy online
pharmacy wholesalers canada: canadian pharmacy online – canadian online drugs
best canadian pharmacy no prescription online pharmacy mail order prescription drugs from canada
canadian pharmacy world reviews canada pharmacy online canadian online drugs
http://pharmworld.store/# canadian pharmacy coupon code
buying prescription drugs online canada: buy drugs online without prescription – online canadian pharmacy no prescription
best online canadian pharmacy: online canadian pharmacy – recommended canadian pharmacies
https://pharmcanada.shop/# canadian online drugs
top online pharmacy india online shopping pharmacy india mail order pharmacy india
pharmacy rx world canada my canadian pharmacy canadian pharmacy online ship to usa
canadian pharmacy near me: canadian pharmacy – pharmacy canadian superstore
http://pharmworld.store/# canadian pharmacy no prescription needed
cheap pharmacy no prescription: cheapest pharmacy – canadian pharmacy no prescription needed
buying from online mexican pharmacy: buying prescription drugs in mexico online – mexican drugstore online
mail order pharmacy india: indian pharmacy – pharmacy website india
canada ed drugs: pharmacy canadian superstore – reputable canadian online pharmacies
Online medicine order world pharmacy india online shopping pharmacy india
canadian drugstore online canadian pharmacies online canadian drugs
http://pharmmexico.online/# buying from online mexican pharmacy
https://pharmnoprescription.icu/# cheap prescription drugs online
online drugs no prescription: canada prescriptions by mail – best online pharmacy no prescription
best canadian pharmacy to order from: canadian pharmacy meds – canadian drug stores
mexican mail order pharmacies: mexican online pharmacies prescription drugs – mexican pharmaceuticals online
п»їbest mexican online pharmacies buying prescription drugs in mexico online mexico drug stores pharmacies
https://pharmworld.store/# non prescription medicine pharmacy
online pharmacy no prescription canadian pharmacy no prescription required canada prescriptions by mail
non prescription online pharmacy india: canada pharmacy without prescription – purchasing prescription drugs online
how to order prescription drugs from canada: no prescription needed – online drugs no prescription
http://pharmnoprescription.icu/# online meds without prescription
https://pharmindia.online/# indian pharmacies safe
india pharmacy mail order reputable indian pharmacies top online pharmacy india
mexican prescription drugs online canadian pharmacy no prescription required no prescription needed
http://pharmworld.store/# canadian pharmacy coupon
online pharmacy discount code: non prescription medicine pharmacy – best canadian pharmacy no prescription
canadian pharmacy world coupons: pharm world store – cheapest pharmacy for prescriptions without insurance
best website to buy prescription drugs: online pharmacy without prescription – online pharmacies without prescription
canadian pharmacy sarasota: canadian drug stores – canadian pharmacy meds
http://pharmcanada.shop/# canadian pharmacies compare
doxycycline hyc buy doxycycline online without prescription order doxycycline
canada where to buy neurontin neurontin 200 mg neurontin over the counter
http://zithromaxa.store/# can you buy zithromax over the counter in mexico
can i buy zithromax online: generic zithromax medicine – buy zithromax no prescription
neurontin 100mg tablets: neurontin 4000 mg – neurontin price south africa
http://zithromaxa.store/# zithromax 500 mg
generic doxycycline: where can i get doxycycline – buy doxycycline online without prescription
amoxicillin 500mg prescription: amoxicillin 500 mg price – amoxicillin 875 125 mg tab
cheap prednisone 20 mg prednisone 40mg prednisone 5mg coupon
amoxicillin 500mg price in canada: buy amoxicillin 250mg – amoxicillin 30 capsules price
https://gabapentinneurontin.pro/# medicine neurontin capsules
875 mg amoxicillin cost: amoxicillin 500 mg brand name – amoxicillin discount coupon
zithromax canadian pharmacy: generic zithromax over the counter – zithromax capsules
generic for amoxicillin: how much is amoxicillin prescription – order amoxicillin 500mg
https://gabapentinneurontin.pro/# neurontin sale
prednisone tablets canada: mail order prednisone – prednisone 40mg
prednisone in mexico: where to buy prednisone uk – 40 mg daily prednisone
zithromax cost canada: where can i get zithromax – where to buy zithromax in canada
zithromax z-pak price without insurance: buy zithromax without prescription online – buy zithromax online
neurontin 800: purchase neurontin – cost of brand name neurontin
doxycycline 500mg: buy doxycycline online without prescription – doxycycline 50 mg
neurontin pills: neurontin tablets 300mg – 32 neurontin
can you buy zithromax over the counter in australia zithromax cost zithromax 250 mg
doxycycline without prescription doxycycline vibramycin where to purchase doxycycline
zithromax cost australia: zithromax purchase online – where to buy zithromax in canada
http://prednisoned.online/# prednisone without prescription 10mg
https://prednisoned.online/# prednisone pill 20 mg
amoxicillin capsules 250mg: amoxicillin 250 mg capsule – amoxicillin 500mg cost
neurontin for sale online neurontin uk neurontin sale
buy amoxil amoxicillin 500mg buy online canada amoxicillin tablets in india
order doxycycline: doxycycline 100mg capsules – buy doxycycline without prescription
zithromax capsules: buy zithromax online with mastercard – zithromax online usa no prescription
http://zithromaxa.store/# zithromax cost uk
http://zithromaxa.store/# zithromax online pharmacy canada
doxycycline monohydrate: doxycycline without prescription – doxycycline 100mg dogs
amoxil pharmacy: prescription for amoxicillin – 875 mg amoxicillin cost
amoxicillin capsules 250mg where to get amoxicillin over the counter over the counter amoxicillin canada
neurontin 2018 neurontin brand name neurontin 300 mg cap
buy cheap amoxicillin online: amoxicillin 500mg prescription – amoxicillin online without prescription
doxylin: doxycycline vibramycin – doxycycline 100mg price
http://doxycyclinea.online/# doxycycline 100mg online
https://gabapentinneurontin.pro/# generic neurontin
buy azithromycin zithromax zithromax online paypal can you buy zithromax over the counter
generic amoxicillin online order amoxicillin online amoxicillin 500mg capsules antibiotic
where can you buy zithromax: zithromax cost – zithromax 500
zithromax z-pak price without insurance: zithromax online – zithromax online paypal
where can i get doxycycline: buy doxycycline hyclate 100mg without a rx – how to buy doxycycline online
http://gabapentinneurontin.pro/# neurontin 900 mg
http://gabapentinneurontin.pro/# neurontin buy from canada
neurontin brand name 800mg best price gabapentin 600 mg neurontin 300 mg cap
buy amoxicillin online mexico amoxicillin 500 mg tablet amoxicillin 500mg over the counter
zithromax 250: zithromax antibiotic – zithromax prescription in canada
neurontin 50mg cost: gabapentin – generic gabapentin
prednisone 10 mg coupon: pharmacy cost of prednisone – prednisone 30 mg tablet
http://doxycyclinea.online/# doxycycline generic
neurontin brand name 800mg best price: neurontin 4000 mg – neurontin 800 mg price
buy doxycycline online: doxycycline without prescription – doxycycline without a prescription
http://zithromaxa.store/# zithromax capsules price
neurontin 4000 mg neurontin singapore neurontin 300 mg
brand name neurontin neurontin price comparison neurontin cost generic
zithromax 500mg: where to get zithromax – zithromax 500
no prescription online prednisone: how much is prednisone 10 mg – medicine prednisone 10mg
https://prednisoned.online/# can i buy prednisone online without prescription
cheap doxycycline online: buy doxycycline monohydrate – 200 mg doxycycline
http://zithromaxa.store/# buy cheap generic zithromax
can you buy prednisone in canada ordering prednisone canadian online pharmacy prednisone
amoxicillin azithromycin amoxicillin generic brand amoxicillin 250 mg capsule
buy doxycycline online without prescription: buy cheap doxycycline – doxycycline 100mg online
amoxicillin 250 mg: amoxicillin tablets in india – amoxicillin cost australia
http://gabapentinneurontin.pro/# neurontin capsule 600mg
prednisone cream: order prednisone – prednisone brand name canada
https://doxycyclinea.online/# doxycycline
buy doxycycline online 270 tabs buy doxycycline online uk doxycycline without prescription
mail order prednisone purchase prednisone 10mg prednisone 30 mg
doxycycline tetracycline: buy doxycycline online uk – doxycycline without prescription
doxycycline hyc 100mg: purchase doxycycline online – generic doxycycline
doxycycline generic: buy cheap doxycycline online – doxycycline medication
zithromax prescription online: where can i buy zithromax medicine – zithromax online paypal
https://zithromaxa.store/# zithromax online australia
http://doxycyclinea.online/# buy doxycycline online without prescription
prednisone 60 mg daily: no prescription online prednisone – prednisone 20mg price in india
amoxicillin 250 mg price in india amoxicillin medicine over the counter buying amoxicillin in mexico
amoxicillin 500 mg without a prescription order amoxicillin online where can you get amoxicillin
zithromax cost uk: zithromax pill – how to get zithromax over the counter
buy amoxicillin 500mg canada: where to buy amoxicillin 500mg – amoxicillin 500mg buy online uk
https://gabapentinneurontin.pro/# neurontin 300 mg pill
where can i order prednisone 20mg: buy cheap prednisone – prescription prednisone cost
http://amoxila.pro/# buy amoxicillin 500mg capsules uk
amoxicillin tablets in india amoxicillin 500 coupon how to get amoxicillin
generic amoxicillin online where can i buy amoxicillin without prec order amoxicillin 500mg
amoxicillin canada price: generic amoxil 500 mg – price for amoxicillin 875 mg
gabapentin 300mg: neurontin 1000 mg – neurontin 600 mg capsule
http://doxycyclinea.online/# buy doxycycline online uk
can you buy prednisone over the counter in canada: prednisone 40 mg rx – prednisone prices
https://doxycyclinea.online/# buy cheap doxycycline
neurontin 600 mg pill: neurontin 150mg – neurontin gel
amoxicillin script amoxicillin online purchase amoxicillin in india
zithromax drug: how to get zithromax online – zithromax for sale online
prednisone pill 10 mg: purchase prednisone canada – canine prednisone 5mg no prescription
http://amoxila.pro/# amoxicillin 500mg tablets price in india
neurontin drug: buy cheap neurontin online – neurontin 202
buy doxycycline: doxy – how to order doxycycline
http://zithromaxa.store/# zithromax 250 mg australia
can i buy zithromax over the counter buy zithromax online buy zithromax online cheap
prednisone online australia 10 mg prednisone tablets prednisone 2.5 mg price
neurontin 300mg capsule: neurontin 3 – neurontin 800 pill
prednisone 10mg cost: prednisone 10mg tabs – where can i get prednisone over the counter
https://gabapentinneurontin.pro/# neurontin 2400 mg
where to purchase doxycycline doxycycline medication generic doxycycline
amoxicillin 500mg capsules antibiotic buy amoxicillin online mexico buy cheap amoxicillin
buy doxycycline online uk: doxycycline generic – doxycycline 50 mg
http://gabapentinneurontin.pro/# neurontin discount
doxy 200: generic doxycycline – buy doxycycline without prescription uk
amoxicillin 500mg without prescription: amoxicillin pharmacy price – buy amoxicillin 500mg uk
buy cheap doxycycline online: buy doxycycline without prescription uk – buy doxycycline without prescription uk
http://prednisoned.online/# prednisone cream brand name
how much is generic neurontin: neurontin over the counter – neurontin 400 mg tablets
buy amoxicillin online no prescription where to get amoxicillin over the counter amoxicillin without prescription
neurontin 2400 mg buy neurontin neurontin 100mg price
neurontin sale: cost of neurontin – neurontin 800 pill
http://prednisoned.online/# otc prednisone cream
doxycycline generic: generic doxycycline – generic doxycycline
http://mexicanpharmacy1st.com/# mexico pharmacies prescription drugs
best mexican online pharmacies: mexican pharmacy – purple pharmacy mexico price list
mexican drugstore online mexican drugstore online purple pharmacy mexico price list
buying prescription drugs in mexico online mexican pharmacy mexico drug stores pharmacies
pharmacies in mexico that ship to usa: mexico pharmacy – mexican pharmacy
https://mexicanpharmacy1st.shop/# medicine in mexico pharmacies
mexican online pharmacies prescription drugs: best online pharmacies in mexico – mexico drug stores pharmacies
https://mexicanpharmacy1st.com/# reputable mexican pharmacies online
pharmacies in mexico that ship to usa: medication from mexico pharmacy – mexico drug stores pharmacies
purple pharmacy mexico price list: mexico drug stores pharmacies – medication from mexico pharmacy
https://mexicanpharmacy1st.com/# mexican pharmaceuticals online
http://mexicanpharmacy1st.com/# mexican pharmaceuticals online
mexican pharmacy: purple pharmacy mexico price list – mexico pharmacy
mexican drugstore online medicine in mexico pharmacies buying prescription drugs in mexico online
mexico drug stores pharmacies buying prescription drugs in mexico mexican drugstore online
https://mexicanpharmacy1st.shop/# medicine in mexico pharmacies
mexican border pharmacies shipping to usa: mexico drug stores pharmacies – buying from online mexican pharmacy
mexican border pharmacies shipping to usa best online pharmacies in mexico mexican border pharmacies shipping to usa
https://mexicanpharmacy1st.shop/# mexican border pharmacies shipping to usa
http://mexicanpharmacy1st.com/# mexico drug stores pharmacies
https://mexicanpharmacy1st.online/# mexico drug stores pharmacies
mexican drugstore online: mexico drug stores pharmacies – best online pharmacies in mexico
mexico pharmacy mexican border pharmacies shipping to usa buying from online mexican pharmacy
mexico pharmacy: medication from mexico pharmacy – mexican rx online
https://mexicanpharmacy1st.shop/# п»їbest mexican online pharmacies
https://mexicanpharmacy1st.online/# buying prescription drugs in mexico
mexico pharmacies prescription drugs: mexico drug stores pharmacies – medication from mexico pharmacy
mexican border pharmacies shipping to usa: mexican mail order pharmacies – medication from mexico pharmacy
mexico drug stores pharmacies: best online pharmacies in mexico – mexican mail order pharmacies
mexico pharmacies prescription drugs: mexico drug stores pharmacies – mexico drug stores pharmacies
mexico drug stores pharmacies pharmacies in mexico that ship to usa buying prescription drugs in mexico
reputable mexican pharmacies online purple pharmacy mexico price list buying prescription drugs in mexico online
medicine in mexico pharmacies: buying prescription drugs in mexico online – mexico pharmacies prescription drugs
https://mexicanpharmacy1st.com/# buying prescription drugs in mexico
purple pharmacy mexico price list: buying prescription drugs in mexico – mexico drug stores pharmacies
mexican online pharmacies prescription drugs best online pharmacies in mexico pharmacies in mexico that ship to usa
purple pharmacy mexico price list best online pharmacies in mexico mexican drugstore online
https://mexicanpharmacy1st.shop/# mexico pharmacies prescription drugs
https://mexicanpharmacy1st.com/# п»їbest mexican online pharmacies
medicine in mexico pharmacies: mexico drug stores pharmacies – mexican drugstore online
best mexican online pharmacies: mexican pharmaceuticals online – medication from mexico pharmacy
medicine in mexico pharmacies: mexico pharmacies prescription drugs – buying prescription drugs in mexico
mexican border pharmacies shipping to usa mexico drug stores pharmacies buying prescription drugs in mexico
medicine in mexico pharmacies best online pharmacies in mexico mexican pharmaceuticals online
https://mexicanpharmacy1st.online/# mexico drug stores pharmacies
https://mexicanpharmacy1st.com/# mexico drug stores pharmacies
https://mexicanpharmacy1st.com/# mexico drug stores pharmacies
buying prescription drugs in mexico: mexican online pharmacies prescription drugs – mexico pharmacies prescription drugs
mexican mail order pharmacies: mexican online pharmacies prescription drugs – mexican drugstore online
mexican rx online: mexican drugstore online – purple pharmacy mexico price list
medication from mexico pharmacy mexico drug stores pharmacies mexican pharmacy
buying from online mexican pharmacy mexico pharmacies prescription drugs mexican mail order pharmacies
https://mexicanpharmacy1st.shop/# buying from online mexican pharmacy
https://mexicanpharmacy1st.shop/# mexican pharmaceuticals online
mexico pharmacy: buying prescription drugs in mexico online – mexican mail order pharmacies
mexican border pharmacies shipping to usa: mexico drug stores pharmacies – buying prescription drugs in mexico
medication from mexico pharmacy reputable mexican pharmacies online reputable mexican pharmacies online
mexican mail order pharmacies п»їbest mexican online pharmacies mexican rx online
https://mexicanpharmacy1st.online/# mexican drugstore online
https://mexicanpharmacy1st.shop/# purple pharmacy mexico price list
https://mexicanpharmacy1st.online/# buying prescription drugs in mexico
https://clomiphene.shop/# how can i get clomid for sale
Misoprostol 200 mg buy online: purchase cytotec – cytotec buy online usa
lisinopril cheap brand: cheapest price for lisinopril india – prinivil online
http://clomiphene.shop/# get cheap clomid no prescription
lisinopril cost 5mg lisinopril 10 best price lisinopril 20 tablet
buying generic propecia without rx buying generic propecia get propecia pills
https://propeciaf.online/# get generic propecia pill
buy generic neurontin: cheap neurontin online – neurontin for sale online
http://cytotec.xyz/# buy cytotec pills
zestril tablet price: lisinopril 50 mg – lisinopril 2
neurontin cost australia price of neurontin neurontin tablets 100mg
can i order generic clomid online order clomid without a prescription get generic clomid now
neurontin 800 mg: purchase neurontin – neurontin 800 mg tablets best price
http://clomiphene.shop/# how can i get cheap clomid without insurance
buy propecia without prescription: cost propecia – cheap propecia
buy zestril online buy lisinopril 40 mg tablet generic lisinopril 40 mg
cost of neurontin neurontin cap neurontin brand name 800mg
http://clomiphene.shop/# can i get generic clomid no prescription
https://propeciaf.online/# order propecia no prescription
buy cheap propecia no prescription: get cheap propecia without insurance – buying generic propecia online
http://cytotec.xyz/# cytotec pills online
neurontin 600mg: neurontin 400 mg tablets – gabapentin 300mg
Abortion pills online: buy misoprostol over the counter – buy cytotec over the counter
http://clomiphene.shop/# how to buy clomid without a prescription
buy cytotec over the counter: buy cytotec over the counter – cytotec abortion pill
https://propeciaf.online/# propecia pill
buy cytotec: Abortion pills online – buy cytotec over the counter
neurontin 300 mg caps where to buy neurontin neurontin 300 mg price in india
where to get clomid price can i purchase clomid tablets cost of generic clomid without dr prescription
http://lisinopril.club/# where to buy lisinopril 2.5 mg
http://propeciaf.online/# buying generic propecia without rx
lisinopril cost uk: cost of lisinopril 30 mg – lisinopril 10 mg without prescription
http://cytotec.xyz/# Abortion pills online
lisinopril 10 12.55mg: buy lisinopril 2.5 mg – lisinopril 20 mg price in india
neurontin discount prescription drug neurontin neurontin cap 300mg
how to get neurontin cheap neurontin price in india cost of neurontin 100mg
https://cytotec.xyz/# buy cytotec
http://lisinopril.club/# lisinopril 20 mg cost
cost of propecia tablets: buy propecia without dr prescription – buying propecia without a prescription
http://gabapentin.club/# neurontin tablets 300mg
can you buy clomid prices: can you buy clomid prices – can you buy generic clomid online
50 mg lisinopril lisinopril pill buy cheap lisinopril
lisinopril 40 mg coupon lisinopril brand name in usa zestril price
buy misoprostol over the counter: purchase cytotec – Cytotec 200mcg price
zestril 20: lisinopril 10 mg online no prescription – 10mg generic 10mg lisinopril
buy misoprostol over the counter Misoprostol 200 mg buy online buy cytotec pills online cheap
gabapentin: neurontin 800 mg price – neurontin 300 mg tablets
buy cytotec online buy misoprostol over the counter cytotec buy online usa
https://gabapentin.club/# can i buy neurontin over the counter
buy cytotec: buy cytotec online fast delivery – order cytotec online
order cytotec online: cytotec pills buy online – п»їcytotec pills online
https://cytotec.xyz/# buy cytotec in usa
buy cytotec pills online cheap cytotec abortion pill buy cytotec pills
order generic propecia without insurance order cheap propecia without prescription get propecia pills
2000 mg neurontin: neurontin 400mg – canada neurontin 100mg lowest price
reputable mexican pharmacies online mexican border pharmacies shipping to usa best online pharmacies in mexico
http://cheapestandfast.com/# can you buy prescription drugs in canada
medicine in mexico pharmacies: medication from mexico pharmacy – buying prescription drugs in mexico online
http://cheapestandfast.com/# online pharmacy no prescription
https://36and6health.com/# canadian pharmacy no prescription needed
mexican border pharmacies shipping to usa medicine in mexico pharmacies reputable mexican pharmacies online
mexican border pharmacies shipping to usa: buying prescription drugs in mexico – buying from online mexican pharmacy
http://cheapestmexico.com/# mexico pharmacies prescription drugs
https://cheapestindia.com/# best online pharmacy india
http://cheapestindia.com/# legitimate online pharmacies india
online pharmacy canada cheapestcanada.com canadian drugs
https://cheapestindia.com/# buy medicines online in india
https://cheapestcanada.shop/# canada pharmacy online
https://cheapestmexico.shop/# mexican drugstore online
online pharmacy india world pharmacy india top 10 online pharmacy in india
https://cheapestandfast.com/# best website to buy prescription drugs
https://cheapestcanada.com/# canadian medications
canada pharmacy 24h: cheapest canada – adderall canadian pharmacy
https://cheapestmexico.com/# mexico pharmacies prescription drugs
https://cheapestcanada.com/# buy canadian drugs
https://cheapestandfast.shop/# indian pharmacy no prescription
https://cheapestandfast.com/# online pharmacies without prescriptions
buy medications online no prescription cheapest and fast online drugs no prescription
https://36and6health.shop/# international pharmacy no prescription
https://cheapestandfast.shop/# medications online without prescriptions
https://cheapestcanada.com/# canadian pharmacy king
india online pharmacy reputable indian online pharmacy india pharmacy mail order
pharmacy without prescription: cheapest pharmacy – mail order prescription drugs from canada
http://cheapestmexico.com/# mexican border pharmacies shipping to usa
http://36and6health.com/# pharmacy coupons
https://36and6health.shop/# best online pharmacy no prescription
mexican drugstore online: mexican rx online – mexico pharmacies prescription drugs
http://cheapestindia.com/# best india pharmacy
https://cheapestindia.shop/# reputable indian pharmacies
http://cheapestcanada.com/# canadian drug
canadian pharmacy price checker my canadian pharmacy canadian drug stores
farmacia online senza ricetta acquistare farmaci senza ricetta acquisto farmaci con ricetta
Pharmacie Internationale en ligne: Achat mГ©dicament en ligne fiable – pharmacie en ligne france pas cher
Pharmacie en ligne livraison Europe: Pharmacie Internationale en ligne – pharmacie en ligne fiable
online apotheke rezept: europa apotheke – online apotheke
pharmacie en ligne fiable: Achat mГ©dicament en ligne fiable – pharmacie en ligne france fiable
farmacias online baratas: farmacias online seguras – farmacias online seguras
https://eumedicamentenligne.com/# Achat mГ©dicament en ligne fiable
top farmacia online farmacia online senza ricetta top farmacia online
pharmacie en ligne france livraison internationale: pharmacie en ligne fiable – pharmacie en ligne france livraison belgique
gГјnstige online apotheke online apotheke gГјnstig online apotheke deutschland
ohne rezept apotheke: ohne rezept apotheke – online apotheke
online apotheke versandkostenfrei: günstigste online apotheke – apotheke online
farmacia online barcelona: farmacia online 24 horas – farmacias online seguras
farmacia en casa online descuento: farmacia online madrid – farmacia online barata
https://eufarmaciaonline.shop/# farmacias online baratas
pharmacie en ligne sans ordonnance: pharmacies en ligne certifiГ©es – Achat mГ©dicament en ligne fiable
Pharmacie Internationale en ligne pharmacies en ligne certifiГ©es п»їpharmacie en ligne france
Farmacia online più conveniente: Farmacie online sicure – comprare farmaci online con ricetta
farmacias direct farmacia barata farmacia online barata y fiable
farmacias online baratas: farmacia online envÃo gratis – farmacia online envÃo gratis
medikamente rezeptfrei: online apotheke versandkostenfrei – medikamente rezeptfrei
Pharmacie sans ordonnance: Pharmacie sans ordonnance – pharmacie en ligne pas cher
п»їFarmacia online migliore: Farmacia online miglior prezzo – farmacia online piГ№ conveniente
farmacia barata farmacia online barcelona farmacias online seguras
https://eufarmacieonline.com/# acquistare farmaci senza ricetta
farmacie online sicure: migliori farmacie online 2024 – Farmacia online più conveniente
online apotheke versandkostenfrei gГјnstige online apotheke internet apotheke
günstigste online apotheke: ohne rezept apotheke – günstige online apotheke
pharmacie en ligne fiable: Pharmacie sans ordonnance – pharmacie en ligne france fiable
farmacia online envГo gratis: farmacia online envГo gratis – farmacia barata
gГјnstigste online apotheke gГјnstige online apotheke online apotheke rezept
https://eufarmaciaonline.shop/# farmacias online seguras en espaГ±a
farmacias online seguras: farmacia online madrid – farmacia online españa envÃo internacional
top farmacia online farmaci senza ricetta elenco Farmacie on line spedizione gratuita
farmacia online envÃo gratis: farmacia online barata – farmacia online madrid
farmacie online affidabili: acquistare farmaci senza ricetta – п»їFarmacia online migliore
farmacia online senza ricetta: Farmacie online sicure – farmacie online autorizzate elenco
farmacia online piГ№ conveniente: farmacie online sicure – farmacie online autorizzate elenco
farmacia online madrid: farmacias online seguras – farmacia barata
pharmacie en ligne livraison europe vente de mГ©dicament en ligne pharmacie en ligne livraison europe
farmacia barata: farmacias online seguras en espaГ±a – farmacia online barata y fiable
farmacias direct: farmacias online seguras en españa – farmacia online envÃo gratis
gГјnstige online apotheke online apotheke preisvergleich apotheke online
comprare farmaci online all’estero: farmacia online più conveniente – farmacie online affidabili
farmacias direct: farmacias online seguras en espaГ±a – farmacia barata
farmacia online envГo gratis: farmacia online 24 horas – farmacia online espaГ±a envГo internacional
medikament ohne rezept notfall: eu apotheke ohne rezept – gГјnstigste online apotheke
https://eumedicamentenligne.com/# pharmacie en ligne fiable
online apotheke deutschland apotheke online online apotheke gГјnstig
pharmacie en ligne pas cher: pharmacie en ligne fiable Рvente de m̩dicament en ligne
comprare farmaci online con ricetta Farmacie on line spedizione gratuita comprare farmaci online all’estero
pharmacie en ligne livraison europe: pharmacie en ligne france livraison internationale Рtrouver un m̩dicament en pharmacie
farmacia online senza ricetta: acquistare farmaci senza ricetta – Farmacia online piГ№ conveniente
http://eumedicamentenligne.com/# Pharmacie sans ordonnance
farmacie online sicure: farmacia online – farmacie online affidabili
medikamente rezeptfrei medikament ohne rezept notfall online apotheke deutschland
Achat m̩dicament en ligne fiable: Pharmacie Internationale en ligne Рpharmacie en ligne pas cher
medikament ohne rezept notfall online apotheke medikamente rezeptfrei
acheter m̩dicament en ligne sans ordonnance: pharmacie en ligne pas cher Рpharmacie en ligne pas cher
online apotheke gГјnstig: medikamente rezeptfrei – apotheke online
online apotheke versandkostenfrei: europa apotheke – online apotheke versandkostenfrei
pharmacie en ligne: Achat mГ©dicament en ligne fiable – pharmacie en ligne pas cher
acquistare farmaci senza ricetta: farmacia online – п»їFarmacia online migliore
Pharmacie sans ordonnance pharmacies en ligne certifiГ©es trouver un mГ©dicament en pharmacie
internet apotheke: online apotheke rezept – apotheke online
Farmacia online più conveniente: farmacie online affidabili – migliori farmacie online 2024
Farmacia online piГ№ conveniente: farmacie online autorizzate elenco – Farmacie on line spedizione gratuita
https://eufarmaciaonline.shop/# farmacias direct
online apotheke rezept: internet apotheke – online apotheke preisvergleich
farmacia online barata y fiable: farmacias online seguras – farmacia online 24 horas
pharmacie en ligne france fiable vente de mГ©dicament en ligne pharmacie en ligne france fiable
Pharmacie Internationale en ligne: pharmacie en ligne fiable Рacheter m̩dicament en ligne sans ordonnance
internet apotheke: online apotheke versandkostenfrei – online apotheke preisvergleich
https://eufarmacieonline.com/# Farmacia online miglior prezzo
acquistare farmaci senza ricetta Farmacie on line spedizione gratuita farmaci senza ricetta elenco
farmacias online seguras: farmacias online baratas – farmacia online barcelona
Achat mГ©dicament en ligne fiable: pharmacie en ligne – Pharmacie Internationale en ligne
farmacia online barata: farmacias online baratas – farmacia online madrid
acheter mГ©dicament en ligne sans ordonnance: kamagra oral jelly – pharmacie en ligne pas cher
https://levitraenligne.shop/# Achat médicament en ligne fiable
Pharmacie sans ordonnance: Pharmacies en ligne certifiees – acheter mГ©dicament en ligne sans ordonnance
pharmacie en ligne sans ordonnance: Pharmacies en ligne certifiees – trouver un mГ©dicament en pharmacie
Quand une femme prend du Viagra homme: Acheter du Viagra sans ordonnance – Viagra gГ©nГ©rique sans ordonnance en pharmacie
pharmacies en ligne certifiГ©es: pharmacies en ligne certifiГ©es – pharmacie en ligne france livraison internationale
pharmacie en ligne france fiable achat kamagra pharmacie en ligne sans ordonnance
pharmacie en ligne avec ordonnance: cialis generique – Pharmacie en ligne livraison Europe
http://kamagraenligne.com/# pharmacie en ligne france livraison internationale
pharmacie en ligne fiable: kamagra en ligne – pharmacie en ligne pas cher
pharmacie en ligne france livraison belgique: Levitra acheter – Pharmacie sans ordonnance
acheter mГ©dicament en ligne sans ordonnance: cialis prix – pharmacie en ligne livraison europe
acheter mГ©dicament en ligne sans ordonnance п»їpharmacie en ligne france pharmacie en ligne sans ordonnance
Viagra 100mg prix: Meilleur Viagra sans ordonnance 24h – Viagra homme prix en pharmacie sans ordonnance
Viagra 100 mg sans ordonnance: Viagra sans ordonnance 24h – Viagra pas cher paris
pharmacie en ligne france fiable: kamagra livraison 24h – pharmacie en ligne avec ordonnance
Viagra gГ©nГ©rique sans ordonnance en pharmacie: Meilleur Viagra sans ordonnance 24h – Viagra pas cher livraison rapide france
Viagra gГ©nГ©rique sans ordonnance en pharmacie: Acheter du Viagra sans ordonnance – Viagra prix pharmacie paris
pharmacie en ligne france livraison internationale: Cialis sans ordonnance 24h – acheter mГ©dicament en ligne sans ordonnance
pharmacie en ligne france livraison internationale: Levitra sans ordonnance 24h – pharmacie en ligne fiable
Pharmacie Internationale en ligne: kamagra oral jelly – pharmacie en ligne livraison europe
Hello!
This post was created with XRumer 23 StrongAI.
Good luck 🙂
trouver un mГ©dicament en pharmacie: pharmacies en ligne certifiГ©es – Pharmacie en ligne livraison Europe
pharmacie en ligne france fiable: pharmacie en ligne sans ordonnance – pharmacie en ligne
Viagra gГ©nГ©rique pas cher livraison rapide: viagra en ligne – Viagra vente libre pays
vente de mГ©dicament en ligne: levitra generique – pharmacies en ligne certifiГ©es
http://viaenligne.com/# Meilleur Viagra sans ordonnance 24h
Viagra gГ©nГ©rique pas cher livraison rapide: Viagra sans ordonnance 24h – Viagra homme sans ordonnance belgique
Viagra homme prix en pharmacie sans ordonnance: viagra sans ordonnance – SildГ©nafil 100 mg prix en pharmacie en France
Viagra 100 mg sans ordonnance: Viagra sans ordonnance livraison 48h – Viagra sans ordonnance livraison 24h
trouver un mГ©dicament en pharmacie: Medicaments en ligne livres en 24h – pharmacie en ligne france livraison belgique
Hello.
This post was created with XRumer 23 StrongAI.
Good luck 🙂
Pharmacie Internationale en ligne: Pharmacies en ligne certifiees – trouver un mГ©dicament en pharmacie
Quand une femme prend du Viagra homme: viagra en ligne – Viagra prix pharmacie paris
Sildenafil teva 100 mg sans ordonnance: Viagra pas cher paris – Viagra homme prix en pharmacie
pharmacie en ligne: pharmacie en ligne livraison europe – pharmacie en ligne pas cher
https://cenligne.com/# Pharmacie en ligne livraison Europe
pharmacie en ligne avec ordonnance: cialis generique – Pharmacie Internationale en ligne
pharmacie en ligne: kamagra 100mg prix – pharmacie en ligne france pas cher
Acheter Sildenafil 100mg sans ordonnance: Viagra sans ordonnance 24h – Quand une femme prend du Viagra homme
SildГ©nafil 100mg pharmacie en ligne: Viagra sans ordonnance 24h – Viagra en france livraison rapide
pharmacie en ligne avec ordonnance: Acheter Cialis 20 mg pas cher – Achat mГ©dicament en ligne fiable
vente de mГ©dicament en ligne: kamagra livraison 24h – Pharmacie Internationale en ligne
Pharmacie sans ordonnance: Levitra acheter – Achat mГ©dicament en ligne fiable
pharmacie en ligne sans ordonnance: pharmacie en ligne – pharmacie en ligne france pas cher
Viagra homme sans prescription: viagra sans ordonnance – Viagra sans ordonnance pharmacie France
п»їpharmacie en ligne france: pharmacie en ligne pas cher – pharmacie en ligne avec ordonnance
Pharmacie en ligne livraison Europe: pharmacie en ligne france livraison internationale – pharmacie en ligne fiable
pharmacie en ligne france pas cher: kamagra 100mg prix – pharmacie en ligne france livraison belgique
pharmacie en ligne: kamagra gel – pharmacies en ligne certifiГ©es
pharmacie en ligne france pas cher: pharmacies en ligne certifiГ©es – pharmacie en ligne livraison europe
pharmacie en ligne avec ordonnance: pharmacie en ligne sans ordonnance – pharmacie en ligne avec ordonnance
п»їpharmacie en ligne france: kamagra pas cher – Pharmacie Internationale en ligne
Viagra en france livraison rapide: Meilleur Viagra sans ordonnance 24h – Viagra homme prix en pharmacie sans ordonnance
Pharmacie Internationale en ligne: pharmacie en ligne france fiable – pharmacie en ligne pas cher
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne sans ordonnance – pharmacies en ligne certifiГ©es
vente de mГ©dicament en ligne: kamagra pas cher – pharmacie en ligne avec ordonnance
Pharmacie sans ordonnance: Levitra sans ordonnance 24h – pharmacie en ligne livraison europe
pharmacie en ligne livraison europe: cialis sans ordonnance – acheter mГ©dicament en ligne sans ordonnance
Achat mГ©dicament en ligne fiable: levitra generique sites surs – Pharmacie en ligne livraison Europe
Viagra homme prix en pharmacie sans ordonnance: Viagra pas cher paris – Viagra sans ordonnance 24h Amazon
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne – pharmacie en ligne france pas cher
pharmacies en ligne certifiГ©es: Levitra pharmacie en ligne – pharmacie en ligne france fiable
pharmacie en ligne avec ordonnance: cialis sans ordonnance – pharmacie en ligne pas cher
trouver un mГ©dicament en pharmacie: levitra en ligne – pharmacie en ligne livraison europe
pharmacie en ligne: Acheter Cialis – pharmacie en ligne pas cher
pharmacie en ligne avec ordonnance: kamagra oral jelly – pharmacie en ligne france livraison belgique
pharmacie en ligne: Cialis sans ordonnance 24h – pharmacie en ligne pas cher
Achat mГ©dicament en ligne fiable: cialis sans ordonnance – pharmacie en ligne france livraison internationale
Acheter viagra en ligne livraison 24h: Viagra generique en pharmacie – Prix du Viagra en pharmacie en France
Viagra sans ordonnance 24h: viagra sans ordonnance – Sildenafil teva 100 mg sans ordonnance
pharmacie en ligne avec ordonnance: Levitra sans ordonnance 24h – pharmacie en ligne france livraison internationale
Pharmacie en ligne livraison Europe: п»їpharmacie en ligne france – acheter mГ©dicament en ligne sans ordonnance
SildГ©nafil 100 mg sans ordonnance: Meilleur Viagra sans ordonnance 24h – Viagra femme sans ordonnance 24h
pharmacies en ligne certifiГ©es: levitra generique sites surs – Pharmacie Internationale en ligne
acheter mГ©dicament en ligne sans ordonnance: pharmacie en ligne sans ordonnance – Pharmacie sans ordonnance
pharmacies en ligne certifiГ©es: kamagra gel – Pharmacie en ligne livraison Europe
Pharmacie en ligne livraison Europe: cialis sans ordonnance – pharmacie en ligne france fiable
pharmacie en ligne: kamagra pas cher – pharmacie en ligne fiable
pharmacie en ligne: kamagra en ligne – pharmacie en ligne france livraison internationale
Pharmacie sans ordonnance: pharmacie en ligne – pharmacie en ligne france livraison belgique
pharmacie en ligne avec ordonnance: Acheter Cialis – п»їpharmacie en ligne france
Viagra pas cher livraison rapide france: Meilleur Viagra sans ordonnance 24h – SildГ©nafil Teva 100 mg acheter
pharmacie en ligne livraison europe: Levitra sans ordonnance 24h – trouver un mГ©dicament en pharmacie
pharmacie en ligne france fiable: Levitra 20mg prix en pharmacie – Pharmacie en ligne livraison Europe
Viagra Pfizer sans ordonnance: Viagra gГ©nГ©rique pas cher livraison rapide – Viagra femme sans ordonnance 24h
pharmacie en ligne livraison europe: Cialis sans ordonnance 24h – trouver un mГ©dicament en pharmacie
Viagra pas cher paris: Viagra vente libre pays – Viagra homme prix en pharmacie sans ordonnance
Achat mГ©dicament en ligne fiable: kamagra en ligne – pharmacies en ligne certifiГ©es
pharmacie en ligne: Pharmacies en ligne certifiees – Pharmacie sans ordonnance
Pharmacie sans ordonnance: kamagra livraison 24h – Achat mГ©dicament en ligne fiable
pharmacie en ligne france livraison belgique: cialis generique – pharmacie en ligne avec ordonnance
pharmacie en ligne france livraison internationale: Levitra 20mg prix en pharmacie – pharmacies en ligne certifiГ©es
Achat mГ©dicament en ligne fiable: trouver un mГ©dicament en pharmacie – trouver un mГ©dicament en pharmacie
http://kamagraenligne.com/# pharmacie en ligne france
Pharmacie Internationale en ligne: Levitra acheter – pharmacie en ligne avec ordonnance
pharmacie en ligne livraison europe: Levitra sans ordonnance 24h – pharmacie en ligne france livraison belgique
pharmacie en ligne pas cher: Pharmacies en ligne certifiees – trouver un mГ©dicament en pharmacie
п»їpharmacie en ligne france: pharmacie en ligne france livraison belgique – pharmacie en ligne pas cher
http://phenligne.com/# pharmacie en ligne france livraison belgique
pharmacie en ligne france fiable: Cialis sans ordonnance 24h – pharmacie en ligne france livraison internationale
Pharmacie sans ordonnance: Levitra pharmacie en ligne – pharmacie en ligne pas cher
pharmacie en ligne pas cher: kamagra pas cher – pharmacie en ligne france pas cher
pharmacie en ligne france pas cher: Levitra sans ordonnance 24h – Pharmacie Internationale en ligne
trouver un mГ©dicament en pharmacie: levitra generique prix en pharmacie – pharmacie en ligne sans ordonnance
Pharmacie Internationale en ligne: levitra generique prix en pharmacie – Pharmacie Internationale en ligne
acheter mГ©dicament en ligne sans ordonnance: Medicaments en ligne livres en 24h – pharmacie en ligne livraison europe
Viagra 100mg prix: Le gГ©nГ©rique de Viagra – Viagra pas cher inde
pharmacie en ligne france pas cher: kamagra oral jelly – pharmacie en ligne france fiable
Achat mГ©dicament en ligne fiable: pharmacie en ligne pas cher – Pharmacie Internationale en ligne
Viagra homme prix en pharmacie sans ordonnance: viagra en ligne – Acheter Sildenafil 100mg sans ordonnance
acheter mГ©dicament en ligne sans ordonnance: cialis generique – vente de mГ©dicament en ligne
Pharmacie en ligne livraison Europe: Acheter Cialis 20 mg pas cher – trouver un mГ©dicament en pharmacie
Pharmacie en ligne livraison Europe: pharmacie en ligne pas cher – pharmacie en ligne pas cher
pharmacie en ligne france pas cher: Medicaments en ligne livres en 24h – pharmacie en ligne sans ordonnance
vente de mГ©dicament en ligne: Pharmacies en ligne certifiees – trouver un mГ©dicament en pharmacie
pharmacie en ligne pas cher: Levitra pharmacie en ligne – п»їpharmacie en ligne france
Viagra 100mg prix: Meilleur Viagra sans ordonnance 24h – SildГ©nafil 100 mg prix en pharmacie en France
pharmacie en ligne france fiable: levitra en ligne – Pharmacie sans ordonnance
pharmacie en ligne france pas cher: Pharmacies en ligne certifiees – pharmacie en ligne pas cher
Pin Up Azerbaycan ?Onlayn Kazino: Pin-Up Casino – Pin Up Azerbaycan ?Onlayn Kazino
Pin Up Azerbaycan ?Onlayn Kazino: ?Onlayn Kazino – Pin Up Kazino ?Onlayn
https://autolux-azerbaijan.com/# pin-up kazino
https://autolux-azerbaijan.com/# Pin-up Giris
Pin Up Kazino ?Onlayn: Pin Up Azerbaycan – ?Onlayn Kazino
Pin-Up Casino: pin-up kazino – Pin Up Kazino ?Onlayn
https://autolux-azerbaijan.com/# Pin-up Giris
https://autolux-azerbaijan.com/# Pin Up Kazino ?Onlayn
Pin Up Azerbaycan ?Onlayn Kazino: Pin Up Azerbaycan – Pin Up Azerbaycan ?Onlayn Kazino
https://autolux-azerbaijan.com/# Pin Up Azerbaycan
Pin Up Azerbaycan ?Onlayn Kazino: Pin-Up Casino – pin-up kazino
https://autolux-azerbaijan.com/# Pin Up Azerbaycan
?Onlayn Kazino: Pin Up – Pin Up Azerbaycan
https://autolux-azerbaijan.com/# Pin-up Giris
https://autolux-azerbaijan.com/# pin-up360