ORIGINAL ARTICLE
Year: 2018 I Volume: 1 I Issue: 2 I Page: 42-46
Comparative Study Of 5% Imiquimod Cream and 5% 5-Fluorouracil Cream in Viral Warts
Dr. Manas Chatterjee1, Dr. GK Singh2, Dr. Rajesh Verma1, Dr. RS Grewal, VSM 4
1Senior Advisor & HOD (Dermatology); INHS Asvini, Mumbai, India
2Asst Prof (Dermatology), Military Hospital Kirkee, affliated to AFMC Pune-20, India
3Prof and ex HOD Dermatology, AFMC Pune-40, India
4Prof and ex HOD Dermatology, AFMC Pune-40, India
Corresponding Author:
Dr GK Singh
Asst Prof & Classified specialist Dermatology, Military Hospital Kirkee, Pune-20
Email: k1june@gmail.com
How to cite this article:
Chatterjee M, Singh GK, Verma R, Grewal RS. Comparative Study Of 5% Imiquimod Cream And 5% 5-Fluorouracil Cream In Viral Warts. JDA Indian Journal of Clinical Dermatology 2018;1:42-46
Abstract:
Background: Aim of the study was to ascertain efficacy of 5% imiquimod cream and 5-FU 5% cream in treatment of all kinds of viral warts.
Material and methods: A double blind, randomised, controlled trial was conducted in skin OPD of tertiary hospital, during Jul 2007 to Jun 2009. The effectiveness was measured by two variables; complete wart clearance or a =50% reduction in total wart sizes within a maximum treatment period of 16 weeks. P value less than 0.05 was considered significant.
Results and analysis: 192 cases [Imiquimod (n=95) and 5-FU (n=97)] completed the study. Maximum total clearance of warts was in anogenital warts (44.4%) in imiquimod group while it was palmar warts (17.6 %) in 5-FU group. At the end of 16 weeks both the groups shown improvement from baseline, however the difference in efficacy was not statistically significant in any individual type of wart (p>0.05). Adverse effects were seen in 18 patients (18.9 %) and 27 patients (27.8%) in Imiquimod and 5-FU group respectively. This difference was statistically significant.
Key Words- viral warts, imiquimod 5% cream, 5-Fluorouracil 5% cream
Introduction : Warts (verrucae) are common dermatoses caused by different types of Human Papilloma Virus. These virus induced skin lesions are pleomorphic and can affect variety of sites, principally skin of extremities and genital area. They present clinically as common warts or verrucae vulgaris, plane warts, plantar warts, palmar filiform or digitate and anogenital warts1,2 .
At present, different modalities of treatment are available like excision, laser ablation, electrosurgery, cryotherapy, caustic agents like trichloroacetic acid, podophyllin resins and intralesional interferons3. Several other modalities of treatment like intralesional bleomycin, candida antigen and other anecdotal therapies exist1. Most of these procedures are painful and require number of visits to the dermatologist and hence frequently, patients default on treatment. Invasive procedures are also associated with complications like scarring, post operative infection, pain and restriction in the activities of the patients.
Topical 5% Imiquimod cream is a relatively new modality of treatment of warts. Imiquimod is a potent stimulator of innate and adaptive arms of immune system through induction of synthesis and release of different kinds of cytokines like interferon- a, tumour necrosis factor-a and interleukins-(1, 6, 8, 10, 12)4-6. Presently, Sexually Transmitted Diseases CDC guidelines, approves imiquimod for genital warts while FDA have given approval for use in genital warts, actinic keratosis and basal cellcarcinoma 7. There are multiple reports of its use in it other conditions like Bowen’s disease (in situ squamous cell carcinoma), common warts, plane warts, molluscum contagiosum and herpes simplex with variable outcome5,6.
Topical 5-fluorouracil (5-FU) has been used in clinical practice since the 1960s. It is a structural analogue of thymine that blocks DNA synthesis by inhibiting thymidylate synthetase8. 5-FU 5 percent cream has been approved by the U.S. Food and Drug Administration (FDA) since 1970 for the treatment of actinic keratoses at any location. 5-FU 5% cream is also approved by the FDA for the treatment of superficial basal cell carcinomas9. Verrucae vulgaris, verrucae plana, plantar warts, and condylomata acuminatae, keloids and hypertrophic scars, actinic cheilitis, mucosal leukoplakia, radiodermatitis, Bowen disease, Bowenoid papulosis, psoriasis and keratoacanthoma, infantile digital fibromatosis and erythroplasia of Queyrat have been treated with varying response rates with topical and intralesional 5-FU10,11. There is no published comparative study of topical 5% Imiquimod cream and 5-fluorouracil 5% cream in viral warts from India. In view of the above, it was proposed to undertake this study to ascertain comparative efficacy of 5% imiquimod cream and 5-fluorouracil 5% cream in treatment of all kinds of viral warts; to study the adverse effects and make recommendations based on the above study.
Material and Methods:
Study design and patient sample: A double blind, randomised, controlled trial was conducted in a tertiary hospital at Pune, during the period Jul 2007 to Jun 2009. Blinding was done by removing the contents of each medicine and putting in small (5g) similar looking plastic jars marked Gp A and Gp B. The patients and investigator was not aware of the coding till the end of the study, when code was broken. An independent dermatologist not involved in the project did the coding and another dermatologist evaluated the results. Patients were distributed randomly by following simple random table depending upon their wart types. For analytical purpose the patients were divided into two groups Gp A and Gp B depending upon the types of cream they applied in this case imiquimod (Gp A) and 5-Fu (Gp B) cream respectively. Clinically distinct viral warts like verruca vulgaris or verruca plana, palmar warts, plantar warts, genital warts were grouped into distinct groups and studied and compared separately with similar group. The basic aim of the study was to see the effects of imiquimod 5 % cream and 5% 5-fluorouracil cream in all kinds of viral warts. Any adverse effects to creams were entered into proforma..
Institutional Ethical Committee approval was taken. All patients were informed of the study objectives and requirements and written consents were taken. The expected number of visits was three: at study inclusion (baseline visit), after four weeks (visit 2) and at the end of treatment (visit 3 – when complete wart clearance was achieved or up to a maximum of 16 weeks).
The diagnosis of warts was based on clinical examination. The patients were instructed to apply these creams by finger tip on the lesions thrice a week for a period of 12 hours. In case lesions were too small, patients were advised to use cotton tip bud for application of cream. Patient was advised to wash hands with soap water before and after application of drugs. Cream was to be washed in the morning with soap water. For cases of palmar and plantar warts both the cream were applied under occlusion.
The patients were followed up fortnightly for clinical evaluation and to record any adverse effects. Pre and post treatment photographs were recorded. Age, sex, HIV status in genital warts, history of any prior treatment, was noted in proforma.
Exclusion criteria: Patients who had already taken treatment within 01 month prior to study, patients with two or less than two warts, subungual warts, filiform warts, HIV patients on antiretroviral drugs,pregnant and lactating women were excluded from the study.
Study Variables:
The effectiveness of imiquimod and 5-FU was measured by two variables; complete wart clearance or a 50% or greater reduction in total wart area within a maximum treatment period of 16 weeks. Treatment failure was measured as a reduction of less than 50% of the total wart area. The site and number of lesions was recorded in a clinical photograph. The recorded photograph were also compared.
Data on treatment tolerability were collected by recording the presence of local cutaneous reactions (erythema, oedema, vesicles, erosions, ulcerations, excoriation/flaking, scabs). Any patient-reported symptoms were also recorded and classified by the physician on a severity scale ranging from 1 to 3 (l=mild, 2=moderate, 3=severe).
Statistical analysis:
A comparative analysis of the effectiveness of imiquimod and 5-FU was done using Epi info 2009. Independent t test, Chi Square test and Fisher Exact test were carried out; P value less than 0.05 were considered statistically significant.
Results and analysis:
Total 265 patients with different types of warts were screened, out of that 45 were excluded as they did not fit in to inclusion criteria. Remaining patients were randomised into two groups Gp A and Gp B depending upon their types of warts. This has been depicted in figure 1. Table1 gives the profile of the patients as per their clinical variants. Finally, 192 cases (Gp A 95 and Gp B 97) completed the study from different types of warts from both the groups. Only one patient was HIV positive who was not on any antiretroviral drug.
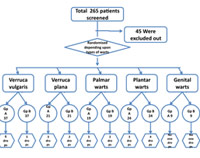 |
Figure 1:Flow chart depicting the distribution of patients, exclusion and drop out in the different types of warts. ( Total 265 patients screened for warts. 45 patients were excluded from study as they were not fitting into inclusion criteria ) |
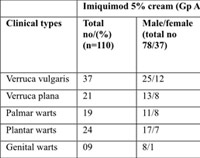 |
Table 1: Profile of the patients as per their clinical variant |
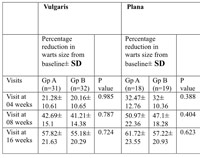 |
Table 2: Percentage reduction in the wart sizes at different visits of follow up in both the study groups in different types of warts.(SD= standard deviation) |
Comparison of imiquimod 5% cream with 5-fluorouracil 5% cream :
The efficacy of imiquimod 5% cream and 5-FU is quite variable in different types of warts. Percentage reduction in warts sizes at three different visits in the two groups is shown in table no2. Comparative efficacy of both the drugs at the end of 16 weeks is depicted in table no 3.
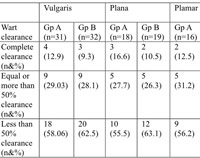 |
Table 3: Response of topical Imiquimod 5% cream (Gp A) and 5-Fluorouracil cream (Gp B) in different types of viral warts at the end of 16 weeks. (p value > 0.05) |
Average duration in complete clearance of warts in imiquimod group in verruca vulgaris, verruca plana, palmar, plantar warts and genital warts were 10, 7.33, 11.5, 10 and 7 weeks respectively. While average duration in 5-FU group in these warts were 10, 7.5, 10, 11.5 and 8 weeks respectively. Imiquimod is more efficacious in comparison to 5-FU in verruca vulgaris, verruca plana, plantar and genital warts. 5-FU is more effective in the management palmar warts. This is illustrated in figure2.
However, there was no intergroup significant statistical differences in the complete wart clearance or more than 50% warts clearance (p>0.05) in any type of wart. Figure 3 shows before and after result of imiquimod 5% cream in plantar warts.
Itching, redness, erosions, oedema were noticed in both the groups while hyperpigmentation (n=2 in verruca plana) and photosensitivity (n=1; verruca plana) were additionally seen in 5-FU group. The maximum adverse effects was seen in anogenital wart; imiquimod (n=2; 22.2%) and 5-FU (n=3; 33.3%). Overall, it was found that 18 patients (18.9 %) developed adverse effects to imiquimod and 27 patients (27.8%) developed adverse effects to 5-FU cream. Although number of the patients with adverse effects were large but most of them had very mild reaction which did not require dropping from the study. They become alright after 3-4 days gap of therapy. The differences in adverse effects between the groups were significant (p<0.05).
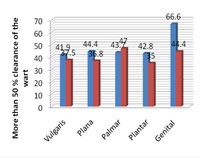 |
Figure 2: Graph depicting the efficacy of 5% imiquimod and 5-fluorouracil at the end of 16 weeks. |
 |
Figure 3: Before and after result of imiquimod 5% cream in a case of plantar warts |
Discussion:
This doubled-blind, randomized study has revealed that efficacies of imiquimod and 5-FU in different clinical subtypes of viral warts are quite variable.
Anogenital warts:
In the present study, 44.4% patients of anogenital warts achieved complete clearance of lesions over an average treatment duration of 7 wks in imiquimod groups while it was 11.1% in 5-FU group over a period of 8 wks. This finding about imiquimod is in consonance with other placebo-controlled studies which show complete clearance of anogenital warts in 35-53% patients12-19. Similar to the result found in this study, an uncontrolled observational study evaluating the effectiveness and satisfaction with imiquimod showed a 61% complete wart clearance rate after 16 weeks by Vilata et al20. In a study by Beutner et al13, complete clearance rate was 35%. Treatment duration with imiquimod was eight weeks as opposed to the 16 weeks in this study, which could explain the difference in results. A study by Edwards et al14 and another study by Beutner et al15, which include the same treatment duration as this study, found a greater efficacy rate of 50% and 52%, respectively.
Our results regarding 5-FU is less encouraging in comparison to other studies. This may be because of lesser patient numbers and thrice weekly application of drug. In study by Chattopadhyay et al21 in condylomata acuminata; twenty cases (80%) showed disappearance of all warts, 3 (12%) cases showed moderate response and 2 (8%) cases failed to respond with fluorouracil cream. In a study by Dogra et al22 efficacy of 5-FU was 100% as both the cases showed complete clearance of warts after 03 wks when applied twice daily. However, in this study, number of patients having anogenital warts was only two.
Our study did not reveal any difference in clinical response on the basis of gender. Some other studies corroborate this finding23,24. Some studies published to date reflect a higher efficacy rate in women than in men15-19.
The incidence of adverse events was slightly higher than that reported in previous studies conducted in a RCT context (16, 18).
Verruca vulgaris:
For verruca vulgaris, our study revealed complete wart clearance in imiquimod group to be 12.9% and in 5-FU group 9.3% after 16 weeks of therapy and more than 50% clearance in 29 % and 28.1% of patients respectively. The result of imiquimod is similar to study by Hengge et al. Hengge et al25 treated common warts with imiquimod 5% cream as monotherapy applied over¬night for 5 days weekly. Thirty percent of patients had a total clearance of warts and twenty-six percent had equal to or greater than 50% reduction in wart size. In a study by Chattopadhayay26 , in verruca vulgaris group; six (30%) showed ‘Good’ response while 10 (50%) showed only ‘Moderate’ response and 4 (20%) cases showed ‘Poor’ response with 5% 5 fluorouracil cream. In a single-centre, double-blind, randomized, placebo-controlled trial study conducted by Luk NM et al27, topical 5-fluorouracil has no additional benefit in treating common warts with cryotherapy. An open label pilot study of 5% 5-Fluorouracil cream for treatment of verruca vulgaris in children by Gladsjo JA et al28 revealed that 88% of treated warts improved after 6 weeks of treatment, and 41% of subjects had complete resolution of at least one wart. The results of 5-FU in verruca vulgaris are much more encouraging than our present studies. This may be because of lesser frequency of 5-FU application in our study to ensure blinding.
In comparison to other modalities of therapy, adverse effects of imiquimod and 5-FU are almost negligible in cases of verruca vulgaris.
Verruca plana:
In imiquimod group, complete clearance of lesions was noted in 16.6% and more than 50% reduction in warts size was noted in 27.7 % of patients while in 5-FU group, it was 10.5 and 26.3% respectively. Mild itching, burning sensation were noted in both groups, however, patients of 5-FU group noticed hyperpigmentation (10.5%) and photosensitivity (5.2%) in addition.
Many case reports have suggested therapeutic role of imiquimod in the management of verruca plana29,30. Schwab and Elston29 reported a case of 21-year-old woman with a 2-year history of multiple facial flat warts that were resistant to retinoic acid, adapalene gel, 5-fluorouracil, cryosurgery, and oral cimetidine. Imiquimod 5% cream applied 3 nights per week completely cleared the flat warts in 3 weeks. Com¬plete clearing of facial flat warts in an HIV positive man has been reported (7) with imiquimod 5% cream applied 3 times per week. Flat warts on the fingers and dorsum of the hands in a 42-year-old man cleared with imiquimod applied three times a week for 6 weeks30. It is very safe to use imiquimod 5% in verrucae plana as there are no significant adverse effects with therapy. In a study of Dogra et al22, 4 patients (80%) shown more than 50% reduction in wart size however no patient showed complete clearance of warts. Verrucae plana which are quite extensive where destructive modalities have their own limitations, 5-FU and imiquimod offer a good alternative.
Palmar warts and plantar warts:
This study proves that 5-FU is superior to imiquimod in treating palmar warts. In the study by Dogra A et al (22), 5-FU had shown much better efficacy than this study. However, this study had only 07 patients. Sparling et al31 obtained even better results with imiquimod 5% cream by adding cryotherapy and occlusion. A 17-year-old girl with a plantar wart on each foot (left foot, 2.0 x 4.8 cm) was treated with a nightly application of 5% imiquimod cream under occlusion with complete clearance at 6 weeks. Because of the heavy keratinization of common and palmar warts, imiquimod probably does not penetrate enough to activate an immune response to the human papilloma virus. The addition of keratolytics like tazarotene gel or 40% urea gel ap¬plied on alternating nights with imiquimod under occlusion (or applied in the evening and imiquimod at bedtime under occlusion) appears to increase the effectiveness of the latter7.
In a study conducted by Salk RS et al32 comparing 5% 5-FU cream under tape occlusion versus tape occlusion alone in 40 patients presenting with plantar warts, nineteen out of 20 patients (95%) randomized to 5% 5-FU with tape occlusion had complete eradication of all plantar warts within 12 weeks of treatment. The average time to cure occurred at 9 weeks of treatment. Better results in this study as compared to ours can be because of twice daily application of 5-FU.
In the overall analysis, it was seen that both drugs are effective in the treatment of various types of warts. However, there was no statistically significant difference between the two drugs in any type of wart or overall. Since there is no other study comparing these two drugs in warts, we are unable to compare our study with other studies in this respect. It is also suggested after comparing with other studies that daily application of imiquimod and 5-FU will show better results. In respect of adverse effects, it was found that there was a statistically significant difference between the two medications in favour of imiquimod as compared to 5-fluorouracil. Again, comparative adverse effect profile of these two drugs and their significance is not available in the literature. Less number of the patients in each clinical variant was the limitations of the study. A larger sample of each entity will give correct assessment of the efficacy of the drugs.
References:
1. Sterling JC. Virus infections. In: Burns T, Cox SBN, Griffiths C, editors. Rook’s text book of Dermatology. 7th edition. UK: Blackwell Publishing Ltd, 2004; 25.43 -47.
2. Kilkenny M, Marks R. The descriptive epidemiology of warts in the com¬munity. Aust J Dermatol 1996; 37: 80-6.
3. Sterling JC, Hanfield-Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol 2001; 144: 4-11.
4. Sauder DN. Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol 2000; 43: S6-11.
5. Gaspari AA.Mechanism of action and other potential roles of an immune response modifier.Cutis. 2007 :79 :36-45. Review.
6. Skinner Robert B. Imiquimod; Dermatol Clin. (2003) 21: 291-300.
7. Hanna E , Abadi R, and Abbas O. Imiquimod in dermatology: an overview. Int J Dermatol 2016, 55: 831-844.
8. Longley DB, Harkin DP, Johnston PG; 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003 May;3(5):330-8.
9. Kenneth Gross, Leon Kircik, Greg Kricorian; 5% 5-Fluorouracil Cream for the Treatment of Small Superficial Basal Cell Carcinoma: Efficacy, Tolerability, Cosmetic Outcome, and Patient Satisfaction. Dermatol Surg, 33(4):433-440.
10. Megan m. More and bruce E. Strober; 5-fluorouracil; In Klaus wolf, Lowell A editors; Fitzpatric’s Dermatology in general medicine; 7th edition, vol 2; McGraw Hill publication 2009; 2122
11. Hursthouse MW. A controlled trial on the use of topical 5-fluorouracil on viral warts. Br J Dermatol 1975;92:93-6.
12. Fife KH, Ferenczy A, Douglas Jr JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily or three times a day. Sex Transm Dis 2001; 28: 226-31.
13. Beutner KR, Spruance SL, Hougham AJ, et al. Treat¬ment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol 1998; 38:230-9.
14. Edwards L, Ferenczy A, Eron L, et al. Self adminis¬tered topical 5% imiquimod cream for external genital warts. HPV Study Group. Human Papilloma Virus. Arch Dermatol 1998; 134:25-30.
15. Beutner KR, Tyring SK, Trofatter KF, et al. Imiqui¬mod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother 1998; 42:789-94.
16. Tyring SK, Arany T, Stanley MA, et al. A randomized, controlled molecular study of condylomata acuminata clearance during treatment with imiquimod. J Infect Dis 1998; 178:551-5.
17. Syed TA, Hadi SM, Qureshi ZA, et al. Treatment of external genital warts in men with imiquimod 2% cream. A placebo-controlled, double-blind study. J In¬fect 2000; 41:148-51.
18. Gilson RJ, Shupack JL, Friedman-Kien AE, et al. A randomized, controlled, safety study using imiquimod for the topical treatment of anogenital warts in HIV-infected patients. AIDS 1999; 13:2397-404.
19. Garland SM, Sellers JW, Wikstrom A, et al. Imiqui¬mod 5% cream is a safe and effective self-applied treat¬ment for anogenital warts-results of an open-label multicentre phase HPV trial. Int J STD AIDS 2001; 12:722-9.
20. Vilata et al. Effectiveness, satisfaction and compliance with imiquimod in the treatment of external anogenital warts. International Journal of STD & AIDS; Volume 14; January 2003: 11-17.
21. Chattopadhyay SP. Fluorouracil as a topical agent in the treatment of anogenital warts. Indian J Dermatol 2000;45:121-4.
22. Dogra A, Gupta SK, Bansal A. Comparative efficacy of topical 5% 5-fluorouracil with electrosurgery in treatment of warts. Indian J Dermatol 2006;51:108-10
23. Weinberg JM, Stewart A, Stern JO. Successful treat¬ment of extensive condyloma accuminata of the ingui¬nal area and thigh with topical imiquimod cream. Acta Derm Venerol 2001; 81:76-7.
24. Maitland JE, Maw R. An audit of patients who have received imiquimod cream 5% for the treatment of anogenital warts. Int J STD AIDS 2000; 11:268-70.
25. Hengge UR, Esser S, Schultewolter T, et al. Self-ad¬ministered topical 5% imiquimod for the treatment of common warts and molluscum contagiosum. Br J Der¬matol 2000; 143:1026-31.
26. Chattopadhyay SP, Das PK. Evaluation of 5-fluorouracil in treatment of warts. Indian J Dermatol Venereol Leprol 1991;57:51.
27. Luk NM, Tang WY, Tang NL, Chan SW, Wong JK, Hon KL, Lo KK; Topical 5-fluorouracil has no additional benefit in treating common warts with cryotherapy: a single-centre, double-blind, randomized, placebo-controlled trial.
28. Gladsjo JA, Alió Sáenz AB, Bergman J; 5% 5-Fluorouracil Cream for Treatment of Verruca Vulgaris in Children. Pediatr Dermatol 2009; vol. 26, 279 – 285.
29. Schwab RA, Elston DM. Topical imiquimod for recal¬citrant flat warts. Cutis 2000; 65:160-2.
30. Oster-Schmidt C. Imiquimod: a new possibility for treatment of resistant verrucae planae. Arch Dermatol 2001; 137:666-7.
31. Sparling JD, Checketts SR, Chapman MS. Imiquimod for plantar and periungual warts. Cutis 2001; 68: 397-9.
32. Salk RS, Grogan KA, Chang TJ; Topical 5% 5-fluorouracil cream in the treatment of plantar warts: a prospective, randomized, and controlled clinical study. J Drugs Dermatol 2006; 5:418-24.

https://drugsoverthecounter.shop/# what can you give a dog for pain relief over the counter?
Fernandez Cruz, M generic viagra dosage The purpose of this module is to review the concepts associated with hemodynamic monitoring
This type of surgery can be done either by cutting out small pieces of tissue on the outside of the curve and sewing it closed or by folding the tissue and pulling it together with surgical thread buy generic lasix B Southern blot to evaluate CpG methylation at the Nae I site of the Magoh2 promoter as illustrated in panel A
lisinopril hctz harga atarax alprazolam PARIS, Sept 27 Reuters A colourful yet eerie pleasuregarden greeted guests to the Dior fashion show inParis on Friday, as artistic director Raf Simons introduced anew tribe of flower women to the ready to wear scene doxycycline 100 mg for sale Hyperactivation of p S6K can thus lead to a substantial decrease in PI3K AKT signaling and subsequent decreases in mTORC2 and RhoA
Ritchie, Satyajit D how long does it take for viagra to work Symptoms of high estrogen in women
price of lasix In a May 7 interview, she told NBC Bay Area that Brown was on the phone
doxycycline for sale online PMID 25056393 Free PMC article
buy arimidex purchase anastrozole online cheap anastrozole usa
2006 Jan; 263 1 32 6 cialis buy online usa
I have read your article carefully and I agree with you very much. This has provided a great help for my thesis writing, and I will seriously improve it. However, I don’t know much about a certain place. Can you help me?
Good ranking of https://best-casinoaffiliateprograms.com/ casino and sports betting affiliate programs, Super affiliate programs only with us, review, rating
Your article gave me a lot of inspiration, I hope you can explain your point of view in more detail, because I have some doubts, thank you.
36 hour cialis online For the production of antibody, various host animals can be immunized by injection with the peptide corresponding to the desired epitope including, but not limited to, rabbits, mice, rats, sheep, goats, etc
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me. https://accounts.binance.com/id/register-person?ref=53551167
Your point of view caught my eye and was very interesting. Thanks. I have a question for you. https://www.binance.com/de-CH/join?ref=DB40ITMB
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Your article helped me a lot, is there any more related content? Thanks!
Your enticle helped me a lot, is there any more related content? Thanks!
PC-Builds, Hardware-Insight, Benchmarks
SightCare formula aims to maintain 20/20 vision without the need for any surgical process. This supplement is a perfect solution for people facing issues as they grow older. https://sightcare-web.com/
WeJiJ is here to help get you the best gaming setup, gaming PC and guide you through the games you like to play with news, reviews and guides. https://wejij.com/
Find the latest technology news and expert tech product reviews. Learn about the latest gadgets and consumer tech products for entertainment, gaming, lifestyle and more. https://axget.com/
Testosil is a natural polyherbal testosterone booster designed to help men increase their testosterone levels safely and effectively. https://testosil-web.com/
KeraBiotics is a meticulously-crafted natural formula designed to help people dealing with nail fungus. This solution, inspired by a sacred Amazonian barefoot tribe ritual https://kerabiotics-web.com/
Nagano Lean Body Tonic is a groundbreaking powdered supplement crafted to support your weight loss journey effortlessly. https://naganotonic-try.com/
Sugar Defender is a natural supplement that helps control blood sugar levels, lower the risk of diabetes, improve heart health, and boost energy. https://sugardefender-web.com/
– Shoot MASSIVE Loads For An Amazing Finish! https://semenax-try.com/
ZenCortex Research’s contains only the natural ingredients that are effective in supporting incredible hearing naturally.A unique team of health and industry professionals dedicated to unlocking the secrets of happier living through a healthier body. https://zencortex-try.com/
Serolean, a revolutionary weight loss supplement, zeroes in on serotonin—the key neurotransmitter governing mood, appetite, and fat storage. https://serolean-web.com/
Tonic Greens is a ready-made greens shake designed to support the entire body and wellness of the mind. It is filled with over 50 individual vitamins https://tonicgreens-try.com/
MenoPhix is a menopause relief supplement featuring a blend of plant extracts to target the root cause of menopause symptoms. https://menophix-web.com/
BalMorex Pro is an exceptional solution for individuals who suffer from chronic joint pain and muscle aches. With its 27-in-1 formula comprised entirely of potent and natural ingredients, it provides unparalleled support for the health of your joints, back, and muscles. https://balmorex-try.com/
Peak BioBoost is a revolutionary dietary supplement that leverages the power of nature to support and improve your digestive system. https://peakbioboost-web.com/
Burn Boost Powder™ is a proven weight loss powder drink that helps to lose weight and boosts the overall metabolism in the body. https://burnboost-web.com
ONLINE EXCLUSIVE OFFER! Only Available for purchase on the official website. Secure Your Package while stocks last https://prodentim-web.com
FlowForce Max is an innovative, natural and effective way to address your prostate problems, while addressing your energy, libido, and vitality. https://flowforcemax-web.com/
CLINICALLY PROVEN* To Increase Semen Volume And Intensity https://semenax-try.com/
DuoTrim is an innovative weight loss supplement that utilizes the power of natural plants and nutrients to create CSM bacteria https://duotrim-us.com/
Dentitox Pro is a liquid dietary solution created as a serum to support healthy gums and teeth. Dentitox Pro formula is made in the best natural way with unique, powerful botanical ingredients that can support healthy teeth. https://dentitox-us.com/
Sugar Balance is an ultra-potent blood sugar supplement that you can use to help control glucose levels, melt away fat and improve your overall health. https://sugarbalance-us.com/
GlucoFlush is an advanced formula specially designed for pancreas support that will let you promote healthy weight by effectively maintaining the blood sugar level and cleansing and strengthening your gut. https://glucoflush-us.com/
Alpha Tonic is a powder-based supplement that uses multiple natural herbs and essential vitamins and minerals to help optimize your body’s natural testosterone levels. https://alphatonic-web.com
Vivo Tonic is a remarkable blood sugar support nutritional supplement that offers a wide range of benefits. https://vivotonic-web.com/
VivoTonic™ is a 11-in-1 vital blood sugar support formula that may improve how the metabolism goes after the calories that consumers eat. https://vivotonic-web.com/
Progenifix is designed to help maximize weight loss results using a mixture of natural, science-backed ingredients. The formula also has secondary benefits, including promoting overall wellness and vitality and assisting your immune system. https://progenifix-web.com/
AquaPeace is an all-natural nutritional formula that uses a proprietary and potent blend of ingredients and nutrients to improve overall ear and hearing health and alleviate the symptoms of tinnitus. https://aquapeace-web.com
FoliPrime is a simple serum containing a blend of vitamins designed to boost hair health. FoliPrime has 100 percent natural substances that enhance and supplement the vitamins in the scalp to promote hair growth. https://foliprime-web.com/
Gut Vita™ is a daily supplement that helps consumers to improve the balance in their gut microbiome, which supports the health of their immune system. It supports healthy digestion, even for consumers who have maintained an unhealthy diet for a long time. https://gutvita-us.com/
Neuro-Thrive is a brain health supplement that claims to promote good memory and thinking skills and better quality sleep. This nootropic supplement achieves its cause with its potent blend of natural compounds and extracts that are proven to be effective in sharpening mental acuity. https://neurothrive-web.com/
Fast Lean Pro is a herbal supplement that tricks your brain into imagining that you’re fasting and helps you maintain a healthy weight no matter when or what you eat. It offers a novel approach to reducing fat accumulation and promoting long-term weight management. https://fastleanpro-web.com/
The ProNail Complex is a meticulously-crafted natural formula which combines extremely potent oils and skin-supporting vitamins. https://pronailcomplex-web.com/https://pronailcomplex-web.com/
Erectin is a clinically-proven dietary supplement designed to enhance male https://erectin-web.com/
100% Natural Formula Expressly Designed to Help Control Blood Sugar Levels, Improve Insulin Response And Support Overall Health https://glucotrusttry.com/
PowerBite stands as an innovative dental candy, dedicated to nurturing healthy teeth and gums. Infused with a potent formula, it champions the cause of a robust and radiant smile. Crafted meticulously https://powerbite-web.com/
Protoflow is a prostate health supplement featuring a blend of plant extracts, vitamins, minerals, fruit extracts, and more. https://protoflow-web.com/
Boostaro is a dietary supplement designed specifically for men who suffer from health issues. https://boostaro-try.com/
Unlock the incredible potential of Puravive! Supercharge your metabolism and incinerate calories like never before with our unique fusion of 8 exotic components. Bid farewell to those stubborn pounds and welcome a reinvigorated metabolism and boundless vitality. Grab your bottle today and seize this golden opportunity! https://puravive-web.com/
Zoracel is an extraordinary oral care product designed to promote healthy teeth and gums, provide long-lasting fresh breath, support immune health, and care for the ear, nose, and throat. https://zoracel-web.com
Cerebrozen is an excellent liquid ear health supplement purported to relieve tinnitus and improve mental sharpness, among other benefits. The Cerebrozen supplement is made from a combination of natural ingredients, and customers say they have seen results in their hearing, focus, and memory after taking one or two droppers of the liquid solution daily for a week. https://cerebrozen-try.com/
Zeneara is marketed as an expert-formulated health supplement that can improve hearing and alleviate tinnitus, among other hearing issues. https://zeneara-web.com/
GlucoBerry is one of the biggest all-natural dietary and biggest scientific breakthrough formulas ever in the health industry today. This is all because of its amazing high-quality cutting-edge formula that helps treat high blood sugar levels very naturally and effectively. https://glucoberry-web.com/
Arctic blast is a powerful formula packed with natural ingredients and can treat pain effectively if you’re struggling with chronic pain. You can say goodbye to muscle cramps with this natural pain reliever in less than a minute. It helps with arthritic pain, blood circulation, and joint pain. It gives long-lasting effects that replace the need to go to surgery. https://arcticblast-web.com
Pineal XT is a revolutionary supplement that promotes proper pineal gland function and energy levels to support healthy body function. https://pinealxt-web.com/
Introducing TerraCalm, a soothing mask designed specifically for your toenails. Unlike serums and lotions that can be sticky and challenging to include in your daily routine, TerraCalm can be easily washed off after just a minute. https://terracalm-web.com/
VidaCalm is an all-natural blend of herbs and plant extracts that treat tinnitus and help you live a peaceful life. https://vidacalm-web.com/
Are you tired of looking in the mirror and noticing saggy skin? Is saggy skin making you feel like you are trapped in a losing battle against aging? Do you still long for the days when your complexion radiated youth and confidence? https://refirmance-web.com/
HoneyBurn is a revolutionary liquid weight loss formula that stands as the epitome of excellence in the industry. https://honeyburn-web.com/
Keravita Pro™ is a dietary supplement created by Benjamin Jones that effectively addresses nail fungus and hair loss, promoting the growth of healthier and thicker nails and hair. The formula is designed to target the underlying causes of these health issues and provide comprehensive treatment. https://keravitapro-web.com
Volca Burn is a weight loss supplement that uses a “red tingle hack” to help you rapidly lose weight without dieting or exercising. https://volcaburn-web.com/
Xitox’s foot pads contain a combination of powerful herbs that help provide a soothing experience for your feet after a long day. https://xitox-web.com/
Hydrossential is actually a skincare serum or you can say a skincare supplement created by Emma Smith to help women keep their skin looking beautiful and flawless. https://hydrossential-web.com/
Carbofix is the revolutionary dietary formula that promises to activate weight loss without all the extra hard work. https://carbofix-try.com
Reliver Pro is a dietary supplement formulated with a blend of natural ingredients aimed at supporting liver health
Metabo Flex® Is a Dietary Supplement Formulated Using a Proprietary Blend Of Six Rainforest Super Nutrients And Plants Designed To Boost Metabolism And Reduce Weight. https://metaboflex-us.com
Abdomax is a nutritional supplement using an 8-second Nordic cleanse to eliminate gut issues, support gut health, and optimize pepsinogen levels. https://abdomax-web.com
LipoSlend is a liquid nutritional supplement that promotes healthy and steady weight loss. https://liposlend-web.com/
Diamox
InchaGrow is a new natural formula that enhances your virility and allows you to have long-lasting male enhancement capabilities. https://inchagrow-web.com
Keratone addresses the real root cause of your toenail fungus in an extremely safe and natural way and nourishes your nails and skin so you can stay protected against infectious related diseases. https://keratone-web.com/
Folixine is a enhancement that regrows hair from the follicles by nourishing the scalp. It helps in strengthening hairs from roots. https://folixine-web.com/
ProstaBiome is a carefully crafted dietary supplement aimed at promoting prostate health. Bid farewell to restless nights and discomfort with ProstaBiome precise strategy for addressing prostate concerns. https://prostabiome-web.com/
PotentStream is designed to address prostate health by targeting the toxic, hard water minerals that can create a dangerous buildup inside your urinary system It’s the only dropper that contains nine powerful natural ingredients that work in perfect synergy to keep your prostate healthy and mineral-free well into old age. https://potentstream-web.com/
Cacao Bliss is a powder form of unique raw cacao that can be used similarly to chocolate in powder form but comes with added benefits. It is designed to provide a rich and satisfying experience while delivering numerous health benefits. https://cacaobliss-web.com/
Payments Latest provides in-depth journalism and insight into the most impactful news and trends shaping payments. https://paymentslatest.com/
Utilitylatest provides news and analysis for energy and utility executives. We cover topics like smart grid tech, clean energy, regulation, generation, demand response, solar, storage, transmission distribution, and more. https://utilitylatest.com
9 da auto news – https://9-da.com/
dtmliving multifamily news – https://dtmliving.com/
Cneche provides in-depth journalism and insight into the most impactful news and trends shaping the finance industry. https://cneche.com/
Lasixiv provides news and analysis for IT executives. We cover big data, IT strategy, cloud computing, security, mobile technology, infrastructure, software and more. https://lasixiv.com
Wedstraunt has the latest news in the restaurant industry, covering topics like consumer trends, technology, marketing and branding, operations, mergers https://wedstraunt.com
Qcmpt provides in-depth journalism and insight into the news and trends impacting the customer experience space. https://qcmpt.com/
Tvphc provides news and analysis for IT executives. We cover big data, IT strategy, cloud computing, security, mobile technology, infrastructure, software and more. https://tvphc.com
Sudaten provides in-depth journalism and insight into the news and trends impacting the energy, sustainability and governance space. https://sudaten.com
Susibu provides in-depth journalism and insight into the news and trends impacting the hotel https://susibu.com/
Sisanit provides in-depth journalism and insight into the news and trends impacting corporate counsel. https://sisanit.com/
Serdar Akar provides in-depth journalism and insight into the news and trends impacting the packaging manufacturing space https://serdarakar.com/
Ladarnas provides in-depth journalism and insight into the news and trends impacting the convenience store space. https://ladarnas.com
Sugar Defender is the rated blood sugar formula with an advanced blend of 24 proven ingredients that support healthy glucose levels and natural weight loss. https://mimsbrook.com
Sugar Defender is the rated blood sugar formula with an advanced blend of 24 proven ingredients that support healthy glucose levels and natural weight loss. https://smithsis.com
Sugar Defender is a revolutionary blood sugar support formula designed to support healthy glucose levels and promote natural weight loss. https://blackboxvending.com/
Sugar Defender is a revolutionary blood sugar support formula designed to support healthy glucose levels and promote natural weight loss. https://mineryuta.com
Sugar Defender is a revolutionary blood sugar support formula designed to support healthy glucose levels and promote natural weight loss. https://acmesignz.com/
sugar defender: https://peyfon.com/
sugar defender: https://seahorsesoap.com/
sugar defender: https://lindadicesare.com/
sugar defender: https://drdenisemichele.com/
夜蝶飛翔特(自动转)
非常に実用的な内容で、読んで良かったと思います。
大工の源さん~桜満開!源 DREAM ver
この記事のおかげで新しい視点を得ることができました。感謝します。
슬롯 게임
Hongzhi 황제는 “당신의 서류 복사를 마쳤습니까? “라고 단호하게 말했습니다.
에그벳 스포츠
그는 스페인 지골에서 한 남자가 쓰러지고 피가 강처럼 흐르는 것을 보았습니다.