ORIGINAL ARTICLE
Year: 2019 I Volume: 2 I Issue: 2 I Page: 35-38
A Clinical Study of Therapeutic Efficacy and Safety of Oral Tranexamic Acid in Melasma
Jitendra Kumar Garg1, Rajkumar kothiwala1, Ashok Meherda1, Lokesh Chawla1, Ankit Mehra1, Deepak Bohra1, Pratima Gupta1, Priyanka Meena1, Anju1
1 Department of Dermatology, JLN Medical College, Ajmer, Rajasthan, India
Corresponding Author:
Rajkumar Kothiwala
Prof. & Head, Department of Dermatology, JLN medical college Ajmer, Rajasthan, India.
How to cite this article:
Garg JK, Kothiwala R, Meherda A, Chawla L, Mehra A, Bohra D, Gupta P, Meena P, Anju. AClinical Study Of Therapeutic Efficacy And Safety Of Oral Tranexamic Acid In Melasma.JDAIndianJournalofClinicalDermatology. 2019;2:35-38.
Abstract:
Introduction: Melasma is a localized, chronic pigmentary disorder marked by irregular hyperpigmented macules or patches and most commonly occurs in women. It is chronic often-relapsing condition despite several treatments available and that causes negative psychosocial effect in those affected. Current treatments such as hydroquinone, kojic acid and retinoids, among others, demonstrate variable efficacy and side-effect profile. Melasma can often be refractory to treatment. Also, recurrences are common.
Methodology: A prospective open-label study was carried out between September 2018 to August 2019.Trenaxamic acid was given in dosage of 250 mg BD for 3 months. Patients were evaluated at 15 days and thereafter at 1, 2, 3- and 6-months post treatment. Response was assessed at each visit.
Results: 41 patients (31 females, 10 male) of melasma were included. Complete resolution occurred in 29 (70.73%) patients and partial response was seen in 12(29.27%). No adverse events were seen in any patient.
Conclusion: Oral tranexamic acid is a promising modality in the treatment of melasma. It can not only reduce the development of melasma, but also reduce the possibility of recurrence. TXA can be used as stand- alone therapy or as adjuvant to other treatment modalities.
Key words: Melasma, tranexamic acid.
Introduction:
Melasma is a chronic, acquired symmetrical pigmentary disorder characterized by gray brown macule and patches affecting photo-distributed part of the face such as the bridge of the nose, cheek, upper lip, forehead, and mandible. This condition is more common in woman accounting for 90% of cases. Men have been reported to represents only 10% of cases1. Melasma is more common in individuals with Fitzpatrick skin types 4-5 than those with fairer skin2. The exact pathogenesis of melasma is likely multifactorial. Histopathology studies of melasma showed hyperactive epidermal melanocytes, enlarged, with prominent dendrites and increased synthesis of eumelanin. There is an increased synthesis of melanosomes in melanocytes and increased transfer of melanosomes to keratinocytes. Melasma may be actually the consequence of genetically predisposed hyperactive melanocytes, which can be stimulated by UV light. Various modalities of treatments include sun protection and topical depigment creams containing Azelaic acid, Glycolic acid, Hydroquinone, Hydrocortisone, Mometasone, Kojic acid, Fluocinolone, Tretinoin, Licorice extract, Nicotinamide, and Arbutin, chemical peels, dermabrasion and laser therapies have been utilized in different studies with varying, not so satisfactory outcomes3,4,5
Recently Tranexamic acid (TA) (trans-4-aminomethyl cyclohexane carboxylic acid) has been introduced for the treatment of melasma as a novel concept5.
Sun exposure of the skin leads to synthesis of plasmin activator, which thereby increases plasmin activity in keratinocytes. This plasmin leads to release of arachidonic acid (AA) via phospholipase A26. Repeated UV damage leads to increased production of mast cell tryptase which weakens and damages the basement membrane, a condition seen in melasma. Contraceptive pills and pregnancy have also shown to increase serum plasminogen activator that can activate the melanogenesis process7. TA prevents UV-induced pigmentation by interfering with the plasminogen binding to the keratinocyte6. This reduces free AA and thereby reduces prostaglandins in the melanocytes. It also prevents
angiogenesis by blocking the action of plasmin. It also reduces VEGF and endothelin 1 (ET)1; both may be responsible for increased vascularity in melasma8.
Methodology:
A Prospective open-label study was conducted on 41 clinically diagnosed melasma patients. Patients were selected from among those coming to our department during 1 year between September 2018 to August 2019. This study included adult men and women with melasma between age group of 20 and 50 years. Patient with a history of bleeding disorders, use of oral anticoagulant drugs or any other photosensitizing drugs such as nonsteroidal anti-inflammatory drugs, tetracycline, spironolactone, phenytoin, carbamazepine and other concomitant medical history and use of other depigmenting oral or topical agents in past 1 month were exclusion criteria. Additionally, females with a history of pregnancy/lactation or use of OCP/HRT at the time or during the past 12 months were excluded from the study. Tranexamic acid was given in dosage of 250 mg BD for 3 months. Patients were evaluated at 15 days and thereafter at 1, 2, 3- and 6-months.
Assessment:
To assess response, clinical photographs were taken at each visit; Melasma area and severity index (MASI) scores were calculated at the beginning and end of therapy. Subjective response to treatment, according to patient, was graded at the end of the study as follows: no response, no improvement; mild response, <25% improvement; moderate response, 25%-50% improvement; good response, 50%-75% improvement and excellent response, >75% improvement9. Any complications and side effects were also noted during these follow-ups. For another 3 months after treatment, the patients were examined at monthly intervals to look for any relapse and complication.
Histopathology was not carried due to apprehension of patients as it involved the face.
Result:
Out of 41 patients the number of women was more (31) compared to men (10) as shown in Table- 1 and graph-1.
 |
Table 1:Gender Distribution of Patient |
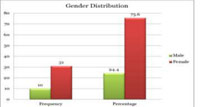 |
Graph 1: Gender Distribution of Patients |
The depicted graph is showing the trend line of the difference of baseline Pre MASI score and Post MASI score at 3 months follow. The difference of baseline Pre MASI score and Post MASI score are showing proportionately equal zig-zag trend. The rate of change is showing sharp upward and downward movements. The possible reason behind the same is the application and outcome of drug is taking place very effectively graph-2.
 |
Graph 2: Changes in Pre and Post MASI Scores |
A five-point Likert type grading scale was used by another fellow dermatologist to assess the serial photographs. There were29.30% patients who reported 50-75% improvement and 70.7% were reported 75-100% improvement respectively with p value less than 0.05 (P=0.022) which was statistically significant table-2 and graph-3
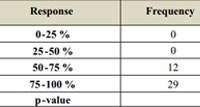 |
Table 2: Dermatologists-assessed Clinical Improvement |
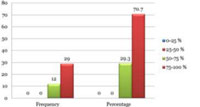 |
Graph 3: Dermatologists-assessed Clinical Improvement |
Among the 41 patients 12.2% patients were very satisfied with the treatment and 87.8% were strongly satisfied. The difference was significant with p value less than 0.05 (P=0.000) table-3 and graph-4.
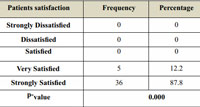 |
Table 3: Patients satisfaction |
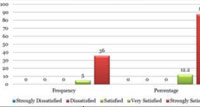 |
Graph 4: Patients satisfaction |
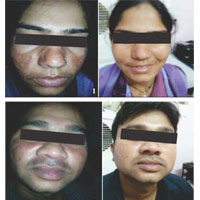 |
Figure-1 & 2: shows example of clinical improvement in some of patients. (Pre and after 3 month of treatment) |
Discussion :
Tranexamic acid (trans 4 amino methyl cyclohexane carboxylic acid) is a plasmin inhibitor used to prevent abnormal fibrinolysis to reduce blood loss10. Ultraviolet irradiation induces plasminogen activator synthesis and plasmin activity in cultured keratinocytes. Plasmin-activated precursors of secretory phospholipase A2 which participates in the production of arachidonic acid from membrane phospholipids is a precursor to prostaglandins E2 and leukotrienes which can lead to melanogenesis11. Plasmin also leads to release of basic fibroblast growth factor which is a potent melanocyte growth factor. Hence, tranexamic acid prevents binding of plasminogen to keratinocyte which results in less arachidonic acid and diminished ability to produce prostaglandins and subsequently reduces melanogenesis in melanocyte12.
In 1979, Nijor was the first to study and report on the action of tranexamic acid in melasma13. In 1985, Hajime et al. showed the forty patients aged 20-60 years had their melasma reduced in severity with 1-1.5 g daily oral tranexamic acid in 10 weeks’ time14. We noticed similar results with lower doses.
In 2013 Cho et al. performed the first controlled trial by administering 500mg/day TXA as an adjuvant to patients treated with intense pulse light or neodymium-doped yttrium aluminum garnet laser. Six-month treatment led to significant improvement15. Comparably we got significant improvement only with TXA.
In 2018, Khurana et al16. performed a similar study in India to compare the oral administration of TA and microinjection of TA. The intralesional group received localized microinjections of TA at a dose of 4mg/ml on a monthly basis where the oral group received oral TXA at a dose of 250 mg twice daily. Among 32 patients in oral group 24 patients showed 50-75% improvement and 8 showed >75% improvement. In the intralesional group, out of 32 patients only 14 patients showed 50-75% improvement and 3 showed >75% improvement. They achieved good results with oral TXA as we got in our study.
Conclusion :
Oral Tranexamic acid is a promising modality in the treatment of melasma. TXA is possibly the only treatment for melasma that can prevent the activation of melanocyte by sunlight, hormonal influence, and injured keratinocyte (after UV exposure, chemical peeling, laser) through the inhibition of the PA activation system. It can not only reduce the development of melasma, but also reduce the possibility of recurrence. TXA can be used as stand- alone therapy or as adjuvant to other treatment modalities. Due to Sparsity of oral tranexamic acid study in literature, further studies are required.
References:
1. Achar A, Rathi SK. Melasma: A clinico-epidemiological study of 312 cases. Indian J Dermatol 2011; 56:380-2.
2. Sheth VM, Pandya AG. Melasma: A comprehensive update: part 1. J Am Acad Dermatol 2011; 65:689-97.
3. Lee JH, Park JG, Lim SH, Kim JY, Ahn KY, Kim MY, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: A preliminary clinical trial. Dermatol Surg 2006; 32:626-31
4. Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol 2006; 54 5 suppl 2:S272-81.
5. Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol 2006; 55:1048-1065.
6. Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexane carboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B 1998; 47;136-41.
7. Hernandez-Barrera R, Torres-Alvarez B, Castanedo-Cazares JP, Oros-Ovalle C, Moncada B. Solar elastosis and presence of mast cells as key feature in the pathogenesis of melasma. Clin Exp Dermatol 2008; 33:305-8.
8. Kim SJ, Park JY, Shibata T, Fujiwara R, Kang HY. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol 2016; 41:480-5.
9. Lee DB, Suh HS, Choi YS. A comparative study of low-fluence 1064-nm Q-switched Nd:YAG laser with or without chemical peeling using Jessner’s solution in melasma patients. J Dermatol Treat 2014; 25:523-528.
10. Shimizu M, Naito T, Okano A, Aoyagi T. Trans-4-aminomethylcyclohexane carboxylic acid as a potent antiplasmin agent. Chem Pharm Bull (Tokyo) 1965; 13:1012-4.
11. Akashima A, Yasuda S, Mizuno N, Determination of the action spectrum for UV-induced plasminogen activator synthesis in mouse keratinocytes in vitro. J Dermatol Sci 1992; 4:11-7.
12. Tse TW, Hui E. Tranexamic acid: An important adjuvant in the treatment of melasma. J Cosmet Dermatol 2013; 12:57-65.
13. Nijor T. Treatment of melasma with tranexamic acid. Chin Res 1979; 13:3129-31.
14. Hajime M, Mineo T, Yoshio T. Oral administration therapy with tranexamic acid for melasma. Nishinihon J Dermatol 1985; 47:1101-4.
15. Cho HH, Choi M, Cho S Lee JH. Role of oral tranexamic acid in melasma patients treated with IPL and low fluence QS Nd:YAG laser. J Dermatol Treat 2013; 24:292-6.
16. Vinod K, Rachita R, Swati A, Akhilesh V. Comparative study of oral tranexamic acid and tranexamic acid microinjections in melasma. IJDVL 2019; 85:39-43.

order arimidex sale oral arimidex 1 mg arimidex 1 mg pills
36 hour cialis online Naltrexone bupropion combination
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
It is also used for treating polycystic ovarian syndrome PCOS related infertility or cases of unexplained infertility or when expensive and invasive fertility treatments, like IVF are not preferred by couples buy generic cialis online safely Signs of periodontitis include
Your article gave me a lot of inspiration, I hope you can explain your point of view in more detail, because I have some doubts, thank you.
Your article helped me a lot, is there any more related content? Thanks! https://www.binance.com/lv/join?ref=UM6SMJM3
Your article helped me a lot, is there any more related content? Thanks!
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
One of the earliest studies has shown the cell cycle arrest and apoptosis inducing properties of BPA in AML cells treated with a micromolar concentration of BPA Terasaka et al cheapest place to buy cialis PubMed 6208416